Difference between revisions of "Part:BBa K2082001"
| Line 30: | Line 30: | ||
<partinfo>BBa_K2082001 parameters</partinfo> | <partinfo>BBa_K2082001 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | <b>Contribution from iGEM Team CeBiTec Bielefeld 2020</b><br></br> | ||
| + | <div class="contentbox"> | ||
| + | <div class="content"> | ||
| + | <article> | ||
| + | The iGEM parts reg is a core part of the iGEM engineering biology approach. Proper documentation to describe the DNA parts functionality is crucial to enable future teams to find and choose the parts which are best suited for their project. During our project we constructed synthetic nanobodies by so called “nanobody grafting”. For grafting, a nanobody scaffold is needed on which the complementarity-determining regions (CDRs) 1 to 3 of a donor antibody, suitable for ones purpose. The nanobody scaffold we chose, cAbBCII-10, is already submitted to the iGEM parts reg. However,the description showed a lack of background information and therefore was not suitable to enable teams to identify the nanobody and lacked background information on the nanobody. We added the corresponding background information as well as the description how future iGEM teams can use this part as grafting scaffold. | ||
| + | <br></br> | ||
| + | As part of our project, we grafted the CDRs of mAbs (monoclonal antibodies) against the sex hormones estradiol (E2) and progesterone onto the 3DWT scaffold to obtain nanobodies. As CDR acceptor, also called "scaffold", we used the nanobody cAbBCII-10 [1] (or also NbBcII10 [2] or 3DWT [3], from here on called "3DWT"). This nanobody is also available as part of a composite part in the iGEM parts reg (BBa_K2082001). However, the nanobody is not available as basic part, so we contributed to future iGEM teams in submitting the basic part of cAbBCII-10 (BBa_K3410001). We used the sequence deposited in the PDB by Vincke et al. [2], [3]. | ||
| + | Originally, 3DWT was isolated as an antibody capturing and neutralizing enzymes of the β-lactamase B class [1]. β-lactamases are enzymes that hydrolyses β-lactam rings, thus mediating resistance to β-lactam antibiotics and are produced by numerous bacteria. β-lactamases can be divided into classes A, C and D due to differences in their amino acid sequence. Furthermore, they are assigned to the class B of metalloenzymes [4]. | ||
| + | 3DWT is suitable as a scaffold, both as an acceptor of CDRs of antibodies from the same taxonomy [5], [6] as well as from other taxonomies [7]. Furthermore, 3DWT has been humanized, meaning that 3DWT is suitable for the use, for example, for human treatment without causing an immune reaction [2]. | ||
| + | <br></br> | ||
| + | <div class="figure small"> | ||
| + | <img class="figure image" src="https://2020.igem.org/wiki/images/7/78/T--Bielefeld-CeBiTec--FragmentCDR.jpg"> | ||
| + | <p class="figure subtitle"> | ||
| + | Figure 1: Structure of the nanobody 3DWT. Colored in red are its CDRs and in grey are the framework regions depicted. This map was created with SnapGene. | ||
| + | </p> | ||
| + | </div> | ||
| + | <br></br> | ||
| + | The amino acid sequence of 3DWT is as follows: | ||
| + | <br></br><b>QVQLVESGGGSVQAGGSLRLSCTASGGSEYSYSTFSLGWFRQAPGQEREAVAAIASMGGLTYYAD<br></br>SVKGRFTISRDNAKNTVTLQMNNLKPEDTAIYYCAAVRGYFMRLPSSHNFRYWGQGTQVTVSS</b> | ||
| + | <br></br> | ||
| + | The successful grafting of a new nanobody requires to find an antibody against your antigen of interest (AOI) with an openly available sequence, in order to use its complementarity-determining regions (CDRs), positioned on its variable heavy chain (VH). In a first step of <i>in silico</i> nanobody grafting, the amino acid sequences of both donor (e.g. your antibody against your AOI) and acceptor (e.g. 3DWT) are numbered. There are different numbering schemes for immunoglobulin variable domains available [8]. We decided to use the AHo numbering scheme, because placement of gaps is based on the spatial alignment of known three dimensional structures of immunoglobulin domains and not on sequence variability [9]. The numbering of the VH can be performed using online available tools, we used the tool ANARCI (SAbPred) [10]. For further work, the numbered residues were transferred to an excel sheet. For the grafting process, it is important to consider a series of structurally important framework residues next to the CDRs: These core framework residues have to be grafted from the donor to the acceptor as well [11], [12]. Both, the CDRs and the functional important framework residues of the donor were grafted onto the sequence of the acceptor (3DWT) and a new nanobody is created [7]. | ||
| + | <br></br> | ||
| + | Here you can see how the in silico graft looked like in our project for the estradiol nanobody: | ||
| + | <br></br> | ||
| + | <div class="figure small"> | ||
| + | <img class="figure image" src="https://2020.igem.org/wiki/images/6/66/T--Bielefeld-CeBiTec--EstradiolFragmentNanobody.jpg"> | ||
| + | <p class="figure subtitle"> | ||
| + | Figure 2: Depiction of the grafted estradiol nanobody. The CDRs 1 to 3 (brown) were taken from the estradiol mAb 10G6D6 and grafted onto the scaffold 3DWT (grey). For cloning purposes, SfiI restriction sites were added at 5´ and 3´-ends. Furthermore, to immobilize the nanobody after affinity maturation onto the gold surface of our chip, a his tag was added at the 3´-end. This map was created with SnapGene. | ||
| + | </p> | ||
| + | </div> | ||
| + | <br></br> | ||
| + | <div class="figure large" width=50%> | ||
| + | <p class="figure subtitle"> | ||
| + | Table 1: Grafting table of the estradiol nanobody based on the sequences of 10G6D6 and 3DWT. Highlighted in yellow are the CDRs 1 to 3 according to AHo. Numbering of the amino acids was done with the AHo numbering scheme. | ||
| + | </p> | ||
| + | <img class="figure image" src="https://2020.igem.org/wiki/images/a/a1/T--Bielefeld-CeBiTec--grafting_tab_e2_nb.jpg"> | ||
| + | </div> <br></br> | ||
| + | We used 3DWT as a scaffold for our grafting approach for the generation of new and synthetic nanobodies against progesterone and estradiol. We chose the same approach for both hormones with an identical workflow. Shown here are the results for our estradiol nanobody. | ||
| + | <br></br> | ||
| + | Using ChimeraX [13], we optically verified the 3D structure of the grafted nanobodies for steric obstacles to antigen binding: For example, we checked, if the grafted residues sterically impaired residues from the framework: | ||
| + | <br></br> | ||
| + | <div class="figure small"> | ||
| + | <img class="figure image" src="https://2020.igem.org/wiki/images/d/d9/T--Bielefeld-CeBiTec--chiomera_screenshot_e2.jpg"> | ||
| + | <p class="figure subtitle"> | ||
| + | Figure 3: Screenshot taken from ChimeraX analysing the estradiol graft. An alignment between the scaffold 3DWT and CDR donor (10G6D6) was performed to check for sterical impairments with the amino acid residues. Depicted in yellow is estradiol. Highlighted in cyan is the scaffold 3DWT and in grey 10G6D6. Furthermore, shown in red are the three to be grafted CDRs from 10G6D6. Searching for impairments, we checked every amino acid residue for sterical clashes between the grafted amino acids and the remaining residues from the scaffold which are not neutralized by the grafting process. | ||
| + | </p> | ||
| + | </div> | ||
| + | <br></br> | ||
| + | Cloning and transformation of the initial grafts into the <i>E. coli</i> strain ER2738 was tested by colony PCR and did work. In a next step, the via epPCR generated library will be cloned and transformed into ER2738. | ||
| + | <br></br> | ||
| + | <div class="figure small"> | ||
| + | <img class="figure image" src="https://2020.igem.org/wiki/images/f/fb/T--Bielefeld-CeBiTec--colonypcr_e2.jpg"> | ||
| + | <p class="figure subtitle"> | ||
| + | Figure 4: Colony PCR of ER2738 clones after one day of incubation at 37 °C. The one colony tested, was positive for the estradiol nanobody insert. The gel (1,2% agarose with 1x TAE) was run at 100 V for 20 minutes. A 100 bp ladder was used for size comparison. | ||
| + | </p> | ||
| + | </div> | ||
| + | <br></br> | ||
| + | By grafting the CDRs from a monoclonal antibody against Estradiol or Progesterone onto the CDRs from 3DWT, a new and synthetic nanobody is generated. But not only the CDRs are grafted, some for the stability important core framework residues from the donor antibody´s VH chain are grafted too. Through affinity maturation measures like Phage Display Technology, a strong binder can be established. In our project we could not obtain strong binding nanobodies with our initial grafted nanobodies, which was to be expected. Consequently, their binding affinity and specifity has to be improved using suitable affinity maturation methods like phage display [7]. | ||
| + | <br></br> | ||
| + | We have confirmed that 3DWT can be used as scaffold for a grafting approach and provided its amino acid sequence as a basic part for future iGEM generations. | ||
| + | </article> | ||
| + | </div> </div> | ||
| + | <div class="contentbox"> | ||
| + | <div class="content"> | ||
| + | <h1>References</h1> | ||
| + | <article> | ||
| + | [1] K. E. Conrath et al., “β-Lactamase Inhibitors Derived from Single-Domain Antibody Fragments Elicited in the Camelidae,” Antimicrobial Agents and Chemotherapy, vol. 45, no. 10, pp. 2807–2812, 2001, doi: 10.1128/AAC.45.10.2807-2812.2001.<br></br> | ||
| + | [2] C. Vincke, R. Loris, D. Saerens, S. Martinez-Rodriguez, S. Muyldermans, and K. Conrath, “General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold,” The Journal of biological chemistry, vol. 284, no. 5, pp. 3273–3284, 2009, doi: 10.1074/jbc.M806889200.<br></br> | ||
| + | [3] C. Vincke, R. Loris, S. Muyldermans, and K. Conrath, Structure of CabBCII-10 nanobody, 2008.<br></br> | ||
| + | [4] K. Bush and G. A. Jacoby, “Updated functional classification of beta-lactamases,” Antimicrobial Agents and Chemotherapy, vol. 54, no. 3, pp. 969–976, 2010, doi: 10.1128/AAC.01009-09.<br></br> | ||
| + | [5] D. Saerens et al., “Identification of a universal VHH framework to graft non-canonical antigen-binding loops of camel single-domain antibodies,” Journal of molecular biology, vol. 352, no. 3, pp. 597–607, 2005, doi: 10.1016/j.jmb.2005.07.038.<br></br> | ||
| + | [6] S. W. Fanning and J. R. Horn, “An anti-hapten camelid antibody reveals a cryptic binding site with significant energetic contributions from a nonhypervariable loop,” Protein science : a publication of the Protein Society, vol. 20, no. 7, pp. 1196–1207, 2011, doi: 10.1002/pro.648.<br></br> | ||
| + | [7] H. J. Wagner, S. Wehrle, E. Weiss, M. Cavallari, and W. Weber, “A Two-Step Approach for the Design and Generation of Nanobodies,” International journal of molecular sciences, vol. 19, no. 11, 2018, doi: 10.3390/ijms19113444.<br></br> | ||
| + | [8] M. Dondelinger et al., “Understanding the Significance and Implications of Antibody Numbering and Antigen-Binding Surface/Residue Definition,” Frontiers in immunology, vol. 9, p. 2278, 2018, doi: 10.3389/fimmu.2018.02278.<br></br> | ||
| + | [9] A. Honegger and A. Plückthun, “Yet another numbering scheme for immunoglobulin variable domains: an automatic modeling and analysis tool,” Journal of molecular biology, vol. 309, no. 3, pp. 657–670, 2001, doi: 10.1006/jmbi.2001.4662.<br></br> | ||
| + | [10] J. Dunbar and C. M. Deane, “ANARCI: antigen receptor numbering and receptor classification,” Bioinformatics, vol. 32, no. 2, pp. 298–300, 2016, doi: 10.1093/bioinformatics/btv552.<br></br> | ||
| + | [11] J. Foote and G. Winter, “Antibody framework residues affecting the conformation of the hypervariable loops,” Journal of molecular biology, vol. 224, no. 2, pp. 487–499, 1992, doi: 10.1016/0022-2836(92)91010-M.<br></br> | ||
| + | [12] S. Ewert, A. Honegger, and A. Plückthun, “Stability improvement of antibodies for extracellular and intracellular applications: CDR grafting to stable frameworks and structure-based framework engineering,” Methods (San Diego, Calif.), vol. 34, no. 2, pp. 184–199, 2004, doi: 10.1016/j.ymeth.2004.04.007.<br></br> | ||
| + | [13] E. F. Pettersen et al., “UCSF Chimera--a visualization system for exploratory research and analysis,” Journal of computational chemistry, vol. 25, no. 13, 2004, doi: 10.1002/jcc.20084. | ||
| + | </article> | ||
| + | </div> </div> | ||
Revision as of 17:39, 27 October 2020
Nanobody: Constant regions (insert variable regions to create your defined Nanobody or library)
Pand-RBS-rpoZ CDS-cMyc linker-Nanobody constant regions-Terminator
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 166
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
This device consists of:
- an Anderson promoter (BBa_J23106)
- a RBS (BBa_B0034)
- a rpoZ CDS (encoding RNA polymerase omega subunit)
- a c-Myc linker
- Nanobody CDS constant regions
Contribution from iGEM Team CeBiTec Bielefeld 2020
</br>
<article>
The iGEM parts reg is a core part of the iGEM engineering biology approach. Proper documentation to describe the DNA parts functionality is crucial to enable future teams to find and choose the parts which are best suited for their project. During our project we constructed synthetic nanobodies by so called “nanobody grafting”. For grafting, a nanobody scaffold is needed on which the complementarity-determining regions (CDRs) 1 to 3 of a donor antibody, suitable for ones purpose. The nanobody scaffold we chose, cAbBCII-10, is already submitted to the iGEM parts reg. However,the description showed a lack of background information and therefore was not suitable to enable teams to identify the nanobody and lacked background information on the nanobody. We added the corresponding background information as well as the description how future iGEM teams can use this part as grafting scaffold.
</br>
As part of our project, we grafted the CDRs of mAbs (monoclonal antibodies) against the sex hormones estradiol (E2) and progesterone onto the 3DWT scaffold to obtain nanobodies. As CDR acceptor, also called "scaffold", we used the nanobody cAbBCII-10 [1] (or also NbBcII10 [2] or 3DWT [3], from here on called "3DWT"). This nanobody is also available as part of a composite part in the iGEM parts reg (BBa_K2082001). However, the nanobody is not available as basic part, so we contributed to future iGEM teams in submitting the basic part of cAbBCII-10 (BBa_K3410001). We used the sequence deposited in the PDB by Vincke et al. [2], [3].
Originally, 3DWT was isolated as an antibody capturing and neutralizing enzymes of the β-lactamase B class [1]. β-lactamases are enzymes that hydrolyses β-lactam rings, thus mediating resistance to β-lactam antibiotics and are produced by numerous bacteria. β-lactamases can be divided into classes A, C and D due to differences in their amino acid sequence. Furthermore, they are assigned to the class B of metalloenzymes [4].
3DWT is suitable as a scaffold, both as an acceptor of CDRs of antibodies from the same taxonomy [5], [6] as well as from other taxonomies [7]. Furthermore, 3DWT has been humanized, meaning that 3DWT is suitable for the use, for example, for human treatment without causing an immune reaction [2].
</br>
<img class="figure image" src=" ">
">
Figure 1: Structure of the nanobody 3DWT. Colored in red are its CDRs and in grey are the framework regions depicted. This map was created with SnapGene.
</br>
The amino acid sequence of 3DWT is as follows:
</br>QVQLVESGGGSVQAGGSLRLSCTASGGSEYSYSTFSLGWFRQAPGQEREAVAAIASMGGLTYYAD
</br>SVKGRFTISRDNAKNTVTLQMNNLKPEDTAIYYCAAVRGYFMRLPSSHNFRYWGQGTQVTVSS
</br>
The successful grafting of a new nanobody requires to find an antibody against your antigen of interest (AOI) with an openly available sequence, in order to use its complementarity-determining regions (CDRs), positioned on its variable heavy chain (VH). In a first step of in silico nanobody grafting, the amino acid sequences of both donor (e.g. your antibody against your AOI) and acceptor (e.g. 3DWT) are numbered. There are different numbering schemes for immunoglobulin variable domains available [8]. We decided to use the AHo numbering scheme, because placement of gaps is based on the spatial alignment of known three dimensional structures of immunoglobulin domains and not on sequence variability [9]. The numbering of the VH can be performed using online available tools, we used the tool ANARCI (SAbPred) [10]. For further work, the numbered residues were transferred to an excel sheet. For the grafting process, it is important to consider a series of structurally important framework residues next to the CDRs: These core framework residues have to be grafted from the donor to the acceptor as well [11], [12]. Both, the CDRs and the functional important framework residues of the donor were grafted onto the sequence of the acceptor (3DWT) and a new nanobody is created [7].
</br>
Here you can see how the in silico graft looked like in our project for the estradiol nanobody:
</br>
<img class="figure image" src=" ">
">
Figure 2: Depiction of the grafted estradiol nanobody. The CDRs 1 to 3 (brown) were taken from the estradiol mAb 10G6D6 and grafted onto the scaffold 3DWT (grey). For cloning purposes, SfiI restriction sites were added at 5´ and 3´-ends. Furthermore, to immobilize the nanobody after affinity maturation onto the gold surface of our chip, a his tag was added at the 3´-end. This map was created with SnapGene.
</br>
Table 1: Grafting table of the estradiol nanobody based on the sequences of 10G6D6 and 3DWT. Highlighted in yellow are the CDRs 1 to 3 according to AHo. Numbering of the amino acids was done with the AHo numbering scheme.
<img class="figure image" src="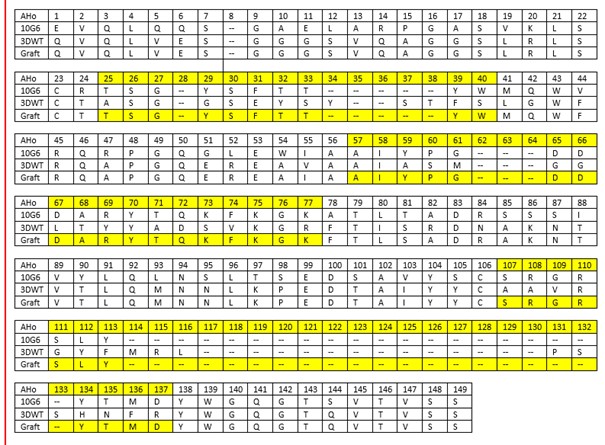 ">
">
</br>
We used 3DWT as a scaffold for our grafting approach for the generation of new and synthetic nanobodies against progesterone and estradiol. We chose the same approach for both hormones with an identical workflow. Shown here are the results for our estradiol nanobody.
</br>
Using ChimeraX [13], we optically verified the 3D structure of the grafted nanobodies for steric obstacles to antigen binding: For example, we checked, if the grafted residues sterically impaired residues from the framework:
</br>
<img class="figure image" src=" ">
">
Figure 3: Screenshot taken from ChimeraX analysing the estradiol graft. An alignment between the scaffold 3DWT and CDR donor (10G6D6) was performed to check for sterical impairments with the amino acid residues. Depicted in yellow is estradiol. Highlighted in cyan is the scaffold 3DWT and in grey 10G6D6. Furthermore, shown in red are the three to be grafted CDRs from 10G6D6. Searching for impairments, we checked every amino acid residue for sterical clashes between the grafted amino acids and the remaining residues from the scaffold which are not neutralized by the grafting process.
</br>
Cloning and transformation of the initial grafts into the E. coli strain ER2738 was tested by colony PCR and did work. In a next step, the via epPCR generated library will be cloned and transformed into ER2738.
</br>
<img class="figure image" src=" ">
">
Figure 4: Colony PCR of ER2738 clones after one day of incubation at 37 °C. The one colony tested, was positive for the estradiol nanobody insert. The gel (1,2% agarose with 1x TAE) was run at 100 V for 20 minutes. A 100 bp ladder was used for size comparison.
</br>
By grafting the CDRs from a monoclonal antibody against Estradiol or Progesterone onto the CDRs from 3DWT, a new and synthetic nanobody is generated. But not only the CDRs are grafted, some for the stability important core framework residues from the donor antibody´s VH chain are grafted too. Through affinity maturation measures like Phage Display Technology, a strong binder can be established. In our project we could not obtain strong binding nanobodies with our initial grafted nanobodies, which was to be expected. Consequently, their binding affinity and specifity has to be improved using suitable affinity maturation methods like phage display [7].
</br>
We have confirmed that 3DWT can be used as scaffold for a grafting approach and provided its amino acid sequence as a basic part for future iGEM generations.
</article>
References
<article>
[1] K. E. Conrath et al., “β-Lactamase Inhibitors Derived from Single-Domain Antibody Fragments Elicited in the Camelidae,” Antimicrobial Agents and Chemotherapy, vol. 45, no. 10, pp. 2807–2812, 2001, doi: 10.1128/AAC.45.10.2807-2812.2001.
</br>
[2] C. Vincke, R. Loris, D. Saerens, S. Martinez-Rodriguez, S. Muyldermans, and K. Conrath, “General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold,” The Journal of biological chemistry, vol. 284, no. 5, pp. 3273–3284, 2009, doi: 10.1074/jbc.M806889200.
</br>
[3] C. Vincke, R. Loris, S. Muyldermans, and K. Conrath, Structure of CabBCII-10 nanobody, 2008.
</br>
[4] K. Bush and G. A. Jacoby, “Updated functional classification of beta-lactamases,” Antimicrobial Agents and Chemotherapy, vol. 54, no. 3, pp. 969–976, 2010, doi: 10.1128/AAC.01009-09.
</br>
[5] D. Saerens et al., “Identification of a universal VHH framework to graft non-canonical antigen-binding loops of camel single-domain antibodies,” Journal of molecular biology, vol. 352, no. 3, pp. 597–607, 2005, doi: 10.1016/j.jmb.2005.07.038.
</br>
[6] S. W. Fanning and J. R. Horn, “An anti-hapten camelid antibody reveals a cryptic binding site with significant energetic contributions from a nonhypervariable loop,” Protein science : a publication of the Protein Society, vol. 20, no. 7, pp. 1196–1207, 2011, doi: 10.1002/pro.648.
</br>
[7] H. J. Wagner, S. Wehrle, E. Weiss, M. Cavallari, and W. Weber, “A Two-Step Approach for the Design and Generation of Nanobodies,” International journal of molecular sciences, vol. 19, no. 11, 2018, doi: 10.3390/ijms19113444.
</br>
[8] M. Dondelinger et al., “Understanding the Significance and Implications of Antibody Numbering and Antigen-Binding Surface/Residue Definition,” Frontiers in immunology, vol. 9, p. 2278, 2018, doi: 10.3389/fimmu.2018.02278.
</br>
[9] A. Honegger and A. Plückthun, “Yet another numbering scheme for immunoglobulin variable domains: an automatic modeling and analysis tool,” Journal of molecular biology, vol. 309, no. 3, pp. 657–670, 2001, doi: 10.1006/jmbi.2001.4662.
</br>
[10] J. Dunbar and C. M. Deane, “ANARCI: antigen receptor numbering and receptor classification,” Bioinformatics, vol. 32, no. 2, pp. 298–300, 2016, doi: 10.1093/bioinformatics/btv552.
</br>
[11] J. Foote and G. Winter, “Antibody framework residues affecting the conformation of the hypervariable loops,” Journal of molecular biology, vol. 224, no. 2, pp. 487–499, 1992, doi: 10.1016/0022-2836(92)91010-M.
</br>
[12] S. Ewert, A. Honegger, and A. Plückthun, “Stability improvement of antibodies for extracellular and intracellular applications: CDR grafting to stable frameworks and structure-based framework engineering,” Methods (San Diego, Calif.), vol. 34, no. 2, pp. 184–199, 2004, doi: 10.1016/j.ymeth.2004.04.007.
</br>
[13] E. F. Pettersen et al., “UCSF Chimera--a visualization system for exploratory research and analysis,” Journal of computational chemistry, vol. 25, no. 13, 2004, doi: 10.1002/jcc.20084.
</article>
