Difference between revisions of "Part:BBa K3457055"
| Line 3: | Line 3: | ||
<partinfo>BBa_K3457055 short</partinfo> | <partinfo>BBa_K3457055 short</partinfo> | ||
| − | a | + | <p> This biological part is the expression sequence of Cytosolic-abundant heat soluble protein 106094, also called CAHS 106094. </p> |
| + | |||
| + | ===Contribution=== | ||
| + | <h3><b>Group: QHFZ-China iGEM 2020</b></h3> | ||
| + | <h3><b>Author: Yixian Yang</b></h3> | ||
| + | <p> TDPs (Tardigrade intrinsically Disordered Proteins) show protective effect to bacteria. However, ralated studies are all use T7 promoter and <i>E. coli</i> BL21 (DE3) strain. This year, we used the Inducible pBad/araC promoter to express a TDP, CAHS 106094, in another starin. For all the experiments below, we use <i>E. coli</i> DH5α strain. The part is constructed by Gibson technique assembly on the basis of [https://parts.igem.org/Part:BBa_K3457037 BBa_K3457037] Arac-Pbad-GFP, which was givren by iGEM Team NEFU-China 2020.</p> | ||
| + | <h3><b>Design</b></h3> | ||
| + | [[File:T--QHFZ-China--ara-222.png|400px|thumb|left|Figure 1. The Schematic cartoon of the DNA construct.]] | ||
| + | <h3 style="clear:left;"><b>Documentation:</b></h3> | ||
| + | <h4 style="clear:left;"><b>Protocol: </b></h4> | ||
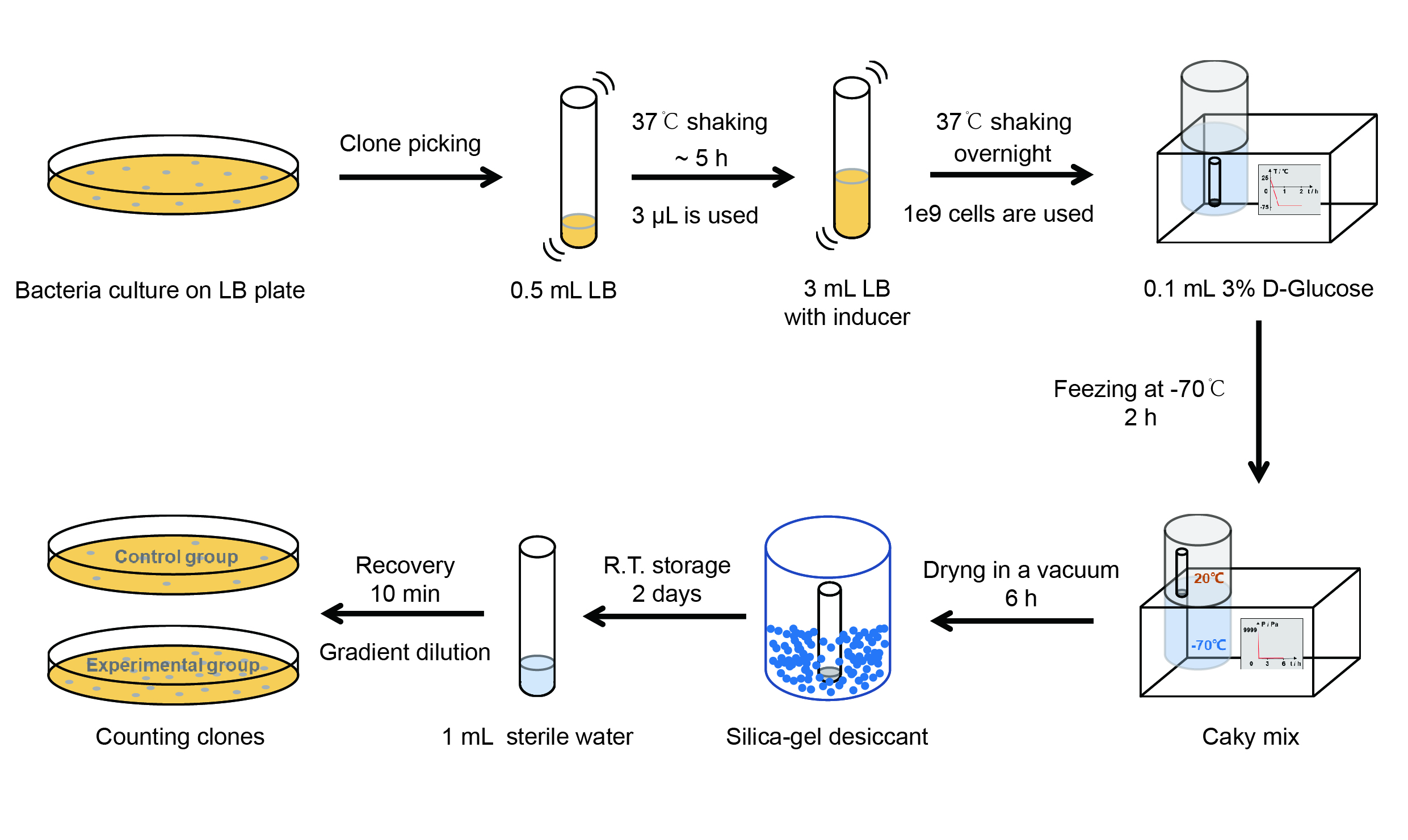
| + | [[File:T--QHFZ-China--freeze-dry protocol.jpg|600px|thumb|left|Figure 2. Experiment protocol.]] | ||
| + | <p style="clear:left;"> | ||
| + | 【Day 1】Induction culture<br> | ||
| + | (1) Pick clones which are in good condition and put them into 500 μL LB medium containing antibiotics. Shake them to | ||
| + | grow at 37℃ for 5~7 hours until the bacteria solution becomes turbid. <br> | ||
| + | (2) Add 0.2% L-arabinose into 3 mL LB medium containing antibiotics. Add 3 μL of the bacteria solution mentioned in step 1 | ||
| + | to dilute the bacteria by the ratio of 1:1000. Shake the solution to grow the bacteria at 37℃ overnight.<br> | ||
| + | 【Day 2】Freeze-dried<br> | ||
| + | (1) If fluorescence induced by the iPTG is detectable in the control group (GFP), continue conducting the | ||
| + | experiment.<br> | ||
| + | (2) Use spectrophotometer to measure the OD<sub>600</sub> of the bacteria solution, OD<sub>600</sub> = 1 equals to | ||
| + | 10<sup>9</sup> cells. If the OD<sub>600</sub> value is between 0.1 and 1, There is a linear relationship between | ||
| + | OD<sub>600</sub> and bacterial density. Calculate the volume of bacterial solution for 10<sup>9</sup> cells by using | ||
| + | the formula V = 100 / (OD<sub>600</sub> × Dilution ratio).<br> | ||
| + | (3) Take out a measured amount of 10<sup>9</sup> cells and centrifuge it at 8000 rpm for 3 min. Then pour out the | ||
| + | supernatant.<br> | ||
| + | (4) Resuspend the bacteria in a 15 mL tube with pre-refrigerated 100 μL 3% glucose solution.<br> | ||
| + | (5) Take off the cover of the tube and put the bacteria into the cold trap. Open the compressor of the | ||
| + | lyophilization machine and freeze the shake tube for 2 h at -70℃.<br> | ||
| + | (6) Put the caky bacteria solution into the drying chamber of the lyophilization machine. Open the vacuum pump to | ||
| + | dry it in vacuum for 6h at 1 Pa vacuum degree.<br> | ||
| + | (7) Turn off the vacuum pump, place it at seal box filled with silica-gel desiccant a for 2 days at room | ||
| + | temperature.<br> | ||
| + | 【Day 3】Room temperature storage<br> | ||
| + | 【Day 4】Detect the survival rate<br> | ||
| + | (1) Add 1 mL of sterile water to the tube, vortex for 15 s, placed it at room temperature for 10 min.<br> | ||
| + | (2) Adjust the density of the bacteria solution by gradient dilution, then spread 100 μL of the bacteria solution on | ||
| + | the LB plate.<br> | ||
| + | (3) If the density above is not suitable, take 100μL of the solution and spread it on the LB plate after several | ||
| + | gradient dilutions.<br> | ||
| + | (4) Culture the bacteria overnight at 37℃.<br> | ||
| + | 【Day 5】Cell Count<br> | ||
| + | (1) Take out the LB plate and take photos to record experimental results.<br> | ||
| + | (2) Use the automatic cell counting function of Image J to count the colone number on the LB plate, then compare the | ||
| + | results between each group.<br> | ||
| + | </p> | ||
| + | <h4>Result</h4> | ||
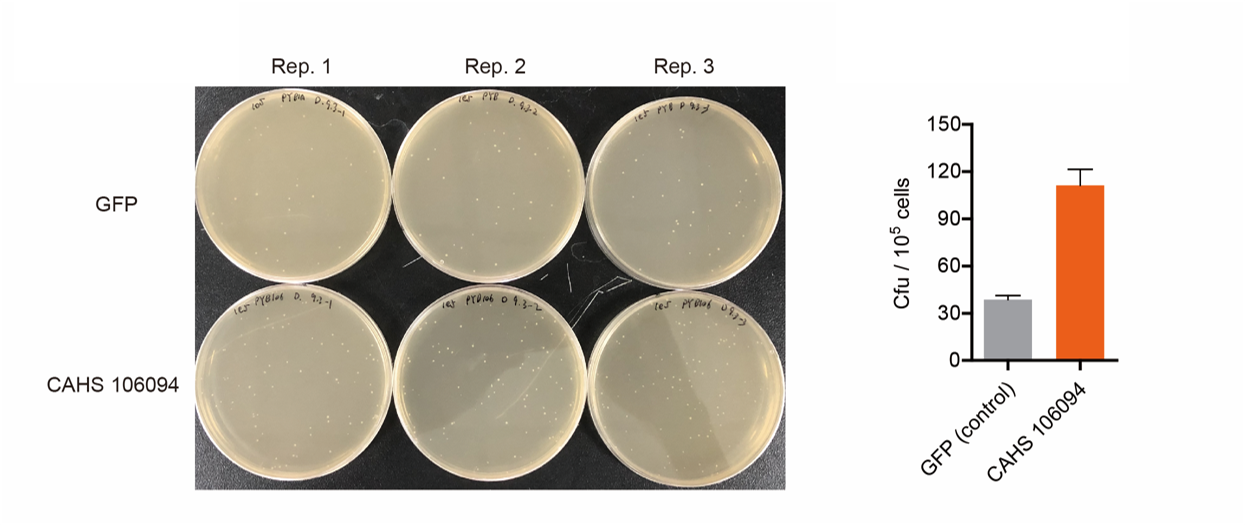
| + | [[File:T--QHFZ-China--ara-4.png|600px|thumb|left|Figure 3. The Cfu of bacteria expressing CAHS 106094 after | ||
| + | freeze-drying.]] | ||
| + | <p style="clear:left;"> As expected, the bacteria with an Arabinose promoter and CAHS 106094 exhibited a higher survival rate, which indicated that the promoter and L-arabinose could successfully induce the expression of CAHS 106094 at enough level in <i>E. coli</i> DH5α strain.</p> | ||
| + | |||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Latest revision as of 03:05, 27 October 2020
Arac-Pbad-CAHS 106094
This biological part is the expression sequence of Cytosolic-abundant heat soluble protein 106094, also called CAHS 106094.
Contribution
Group: QHFZ-China iGEM 2020
Author: Yixian Yang
TDPs (Tardigrade intrinsically Disordered Proteins) show protective effect to bacteria. However, ralated studies are all use T7 promoter and E. coli BL21 (DE3) strain. This year, we used the Inducible pBad/araC promoter to express a TDP, CAHS 106094, in another starin. For all the experiments below, we use E. coli DH5α strain. The part is constructed by Gibson technique assembly on the basis of BBa_K3457037 Arac-Pbad-GFP, which was givren by iGEM Team NEFU-China 2020.
Design
Documentation:
Protocol:
【Day 1】Induction culture
(1) Pick clones which are in good condition and put them into 500 μL LB medium containing antibiotics. Shake them to
grow at 37℃ for 5~7 hours until the bacteria solution becomes turbid.
(2) Add 0.2% L-arabinose into 3 mL LB medium containing antibiotics. Add 3 μL of the bacteria solution mentioned in step 1
to dilute the bacteria by the ratio of 1:1000. Shake the solution to grow the bacteria at 37℃ overnight.
【Day 2】Freeze-dried
(1) If fluorescence induced by the iPTG is detectable in the control group (GFP), continue conducting the
experiment.
(2) Use spectrophotometer to measure the OD600 of the bacteria solution, OD600 = 1 equals to
109 cells. If the OD600 value is between 0.1 and 1, There is a linear relationship between
OD600 and bacterial density. Calculate the volume of bacterial solution for 109 cells by using
the formula V = 100 / (OD600 × Dilution ratio).
(3) Take out a measured amount of 109 cells and centrifuge it at 8000 rpm for 3 min. Then pour out the
supernatant.
(4) Resuspend the bacteria in a 15 mL tube with pre-refrigerated 100 μL 3% glucose solution.
(5) Take off the cover of the tube and put the bacteria into the cold trap. Open the compressor of the
lyophilization machine and freeze the shake tube for 2 h at -70℃.
(6) Put the caky bacteria solution into the drying chamber of the lyophilization machine. Open the vacuum pump to
dry it in vacuum for 6h at 1 Pa vacuum degree.
(7) Turn off the vacuum pump, place it at seal box filled with silica-gel desiccant a for 2 days at room
temperature.
【Day 3】Room temperature storage
【Day 4】Detect the survival rate
(1) Add 1 mL of sterile water to the tube, vortex for 15 s, placed it at room temperature for 10 min.
(2) Adjust the density of the bacteria solution by gradient dilution, then spread 100 μL of the bacteria solution on
the LB plate.
(3) If the density above is not suitable, take 100μL of the solution and spread it on the LB plate after several
gradient dilutions.
(4) Culture the bacteria overnight at 37℃.
【Day 5】Cell Count
(1) Take out the LB plate and take photos to record experimental results.
(2) Use the automatic cell counting function of Image J to count the colone number on the LB plate, then compare the
results between each group.
Result
As expected, the bacteria with an Arabinose promoter and CAHS 106094 exhibited a higher survival rate, which indicated that the promoter and L-arabinose could successfully induce the expression of CAHS 106094 at enough level in E. coli DH5α strain.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 1144
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 979
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI site found at 961