Difference between revisions of "Part:BBa K3570002"
| Line 30: | Line 30: | ||
<b>TDH1</b> promoter proved itself to be quite versatile under different carbon sources for yeast[9]. TDH1 promoter is a gene-specific promoter from the yeast TDH1 gene[10]. The sequence was extracted from TDH1 gene in SGD[10]. <b>CYC1</b> terminator was chosen because of its large usage in yeast biotechnological manipulations[11]. The sequence was identified from the personal communication with Dr. Anthony Henras.</p> | <b>TDH1</b> promoter proved itself to be quite versatile under different carbon sources for yeast[9]. TDH1 promoter is a gene-specific promoter from the yeast TDH1 gene[10]. The sequence was extracted from TDH1 gene in SGD[10]. <b>CYC1</b> terminator was chosen because of its large usage in yeast biotechnological manipulations[11]. The sequence was identified from the personal communication with Dr. Anthony Henras.</p> | ||
| + | <p style="text-indent: 40px"> | ||
| + | <b>HO</b> upstream and downstream homology arms ([https://parts.igem.org/Part:BBa_K3570009 BBa_K3570009] and [https://parts.igem.org/Part:BBa_K3570010 BBa_K3570010]) are used to target a functional yeast integration locus. This will result in homologous recombination within the Diacylglycerol pyrophosphate phosphatase 1 (DPP1) gene and thus integration into the <i>S. cerevisiae</i>'s genome[12]. The sequence was identified from personal communication with Dr. Gilles Truan. | ||
<h2>Experiments</h2> | <h2>Experiments</h2> | ||
| Line 44: | Line 46: | ||
*[10]- [https://www.yeastgenome.org/locus/S000003588 SGD:S000003588] | *[10]- [https://www.yeastgenome.org/locus/S000003588 SGD:S000003588] | ||
*[11]- Curran, K. A., Karim, A. S., Gupta, A., & Alper, H. S. (2013). Use of expression-enhancing terminators in Saccharomyces cerevisiae to increase mRNA half-life and improve gene expression control for metabolic engineering applications. Metabolic Engineering, 19, 88–97. https://doi.org/10.1016/j.ymben.2013.07.001 | *[11]- Curran, K. A., Karim, A. S., Gupta, A., & Alper, H. S. (2013). Use of expression-enhancing terminators in Saccharomyces cerevisiae to increase mRNA half-life and improve gene expression control for metabolic engineering applications. Metabolic Engineering, 19, 88–97. https://doi.org/10.1016/j.ymben.2013.07.001 | ||
| + | *[12]- <i>S. cerevisiae</i> genome, chromosome IV, HO gene. GenBank: CP046084.1 | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Revision as of 15:48, 22 October 2020
Provitamin A synthesis from GGPP in S. cerevisiae
- 10INCOMPATIBLE WITH RFC[10]Illegal XbaI site found at 1297
Illegal PstI site found at 2357
Illegal PstI site found at 2627
Illegal PstI site found at 3605 - 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 2012
Illegal NheI site found at 5006
Illegal PstI site found at 2357
Illegal PstI site found at 2627
Illegal PstI site found at 3605 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 738
Illegal BglII site found at 3364
Illegal BglII site found at 5489
Illegal BglII site found at 6585
Illegal BamHI site found at 3157
Illegal XhoI site found at 4943
Illegal XhoI site found at 4984 - 23INCOMPATIBLE WITH RFC[23]Illegal XbaI site found at 1297
Illegal PstI site found at 2357
Illegal PstI site found at 2627
Illegal PstI site found at 3605 - 25INCOMPATIBLE WITH RFC[25]Illegal XbaI site found at 1297
Illegal PstI site found at 2357
Illegal PstI site found at 2627
Illegal PstI site found at 3605
Illegal NgoMIV site found at 2018 - 1000COMPATIBLE WITH RFC[1000]
Introduction
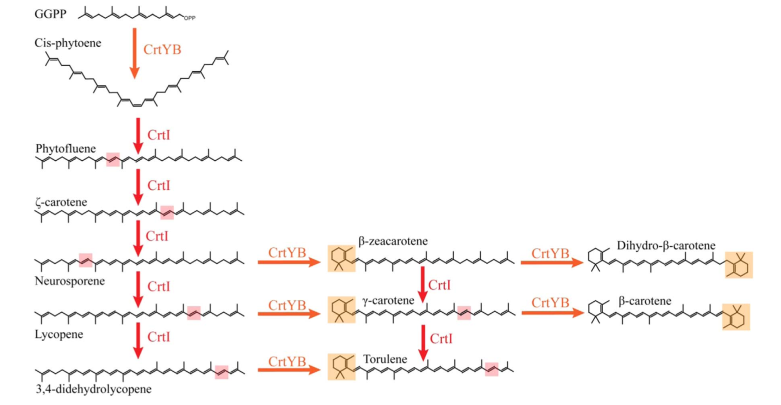
This biobrick shall be used to boost the production of produce provitamin A (𝛽-carotene) in S. cerevisiae . 𝛽-carotene is one of the carotenoids produced in yeast. The metabolic pathway comprises multiple intermediate as well as side-products before reaching 𝛽-carotene (fig. 1). It starts with geranylgeranyl diphosphate (GGPP), which is a derivative from Mevalonate pathway. GGPP is importantly used in yeast since it is a precursor to carotenoids[1], tocopherols[2], and to geranylgeranylated proteins[3]. Therefore, for the best production yield of 𝛽-carotene production using this biobrick, it is best to use it in synergy with the "GGPP production enhancement in S. cerevisiae" biobrick (BBa_K3570000).
Design
Biosynthesis of β-carotene in X. dendrorhous begins with the production of phytoene from GGPP by the domain B of bifunctional lycopene cyclase/phytoene synthase (CrtYB). Phytoene desaturase (CrtI) then catalyzes four successive desaturation reactions to form lycopene. In the end, the domain Y of CrtYB performs the cyclization on both sides of lycopene to produce β-carotene (fig.1).
Eukaryotic CrtY enzymes are predicted to be transmembrane proteins. On the contrary, CrtB and possibly CrtI are supposedly cytosolic[4]. Additionally, in some fungi, the phytoene CrtY and CrtB catalytic activities are fused within a multidomain protein named CrtYB in Xanthophyllomyces dendrorhous[5]. The complete biosynthetic pathway of X. dendrorhous, with a multidomain CrtYB, was efficiently expressed in S. cerevisiae[6]. Even though this heterologous system is the most productive to date, the accumulation of β-carotene precursors in the pathway, such as phytoene, was remarked. Nevertheless, the overexpression of CrtI to overcome the metabolic bottleneck turned out to be only partially successful[6].
Recently, the natural tridomain fusion (CrtIBY) from Schizotrium sp. was expressed in Yarrowia lipolytica[7] but unfortunately, the β-carotene production yields were considerably lower than those obtained with the yeast-expressed X. dendrorhous configuration [6], [8]. We then searched to design an enzyme(s), that would have at the same time high production yields of β-carotene precursors (as in X. dendrorhous) with the spatial proximity of both cytosolic enzymes (CrtB and CrtI).
A strategy for creating an enzyme that would have crtYB et crtI activities and every listed property above was adapted from Rabeharindranto, H et al., 2019. This way, a tridomain fusion protein CrtYBekI was expressed in S. cerevisiae, with ‘‘ek’’ being a peptidic linker. This spatial reorganization of the carotenoid enzymes should reduce the accumulation of intermediates and reorient the metabolic fluxes towards β-carotene production.
TDH1 promoter proved itself to be quite versatile under different carbon sources for yeast[9]. TDH1 promoter is a gene-specific promoter from the yeast TDH1 gene[10]. The sequence was extracted from TDH1 gene in SGD[10]. CYC1 terminator was chosen because of its large usage in yeast biotechnological manipulations[11]. The sequence was identified from the personal communication with Dr. Anthony Henras.
HO upstream and downstream homology arms (BBa_K3570009 and BBa_K3570010) are used to target a functional yeast integration locus. This will result in homologous recombination within the Diacylglycerol pyrophosphate phosphatase 1 (DPP1) gene and thus integration into the S. cerevisiae's genome[12]. The sequence was identified from personal communication with Dr. Gilles Truan.
Experiments
References
- [1]- Rabeharindranto, H., Castaño-Cerezo, S., Lautier, T., Garcia-Alles, L. F., Treitz, C., Tholey, A., & Truan, G. (2019). Enzyme-fusion strategies for redirecting and improving carotenoid synthesis in S. cerevisiae. Metabolic Engineering Communications, 8, e00086
- [2]- DIPLOCK, A. T., GREEN, J., EDWIN, E. E., & BUNYAN, J. (1961). Tocopherol, Ubiquinones and Ubichromenols in Yeasts and Mushrooms. Nature, 189(4766), 749–750. https://doi.org/10.1038/189749a0
- [3]- Ohya, Y., Qadota, H., Anraku, Y., Pringle, J. R., & Botstein, D. (1993). Suppression of yeast geranylgeranyl transferase I defect by alternative prenylation of two target GTPases, Rho1p and Cdc42p. Molecular Biology of the Cell, 4(10), 1017–1025. https://doi.org/10.1091/mbc.4.10.1017
- [4]- Schaub, P., Yu, Q., Gemmecker, S., Poussin-Courmontagne, P., Mailliot, J., McEwen, A.G., et al., 2012. On the structure and function of the phytoene desaturase CRTI from Pantoea ananatis, a membrane-peripheral and FAD-dependent oxidase/ isomerase. PLoS One 7, e39550.
- [5]-Verdoes, J.C., Krubasik, P., Sandmann, G., Van Ooyen, A.J.J., 1999. Isolation and functional characterization of a novel type of carotenoid biosynthetic gene from Xanthophyllomyces dendrorhous. Mol. Gen. Genet. MGG 262, 453–461.
- [6]-Verwaal, R., Wang, J., Meijnen, J.-P., Visser, H., Sandmann, G., Berg, J.A. van den, et al., 2007. High-level production of beta-carotene in saccharomyces cerevisiae by successive transformation with carotenogenic genes from xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 73, 4342–4350.
- [7]-Gao, S., Tong, Y., Zhu, L., Ge, M., Jiang, Y., Chen, D., et al., 2017. Production of βcarotene by expressing a heterologous multifunctional carotene synthase in Yarrowia lipolytica. Biotechnol. Lett. 39, 921–927
- [8]-Xie, W., Ye, L., Lv, X., Xu, H., Yu, H., 2015. Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae. Metab. Eng. 28, 8–18.
- [9]- Monfort, A., Finger, S., Sanz, P., & Prieto, J. A. (1999). Evaluation of different promoters for the efficient production of heterologous proteins in baker's yeast. Biotechnology Letters, 21(3), 225–229. https://doi.org/10.1023/a:1005467912623
- [10]- SGD:S000003588
- [11]- Curran, K. A., Karim, A. S., Gupta, A., & Alper, H. S. (2013). Use of expression-enhancing terminators in Saccharomyces cerevisiae to increase mRNA half-life and improve gene expression control for metabolic engineering applications. Metabolic Engineering, 19, 88–97. https://doi.org/10.1016/j.ymben.2013.07.001
- [12]- S. cerevisiae genome, chromosome IV, HO gene. GenBank: CP046084.1

