Difference between revisions of "Part:BBa K3570002"
| Line 12: | Line 12: | ||
<h2>Design</h2> | <h2>Design</h2> | ||
<p style="text-indent: 40px"> | <p style="text-indent: 40px"> | ||
| − | |||
| − | |||
| − | + | [[File:BBa K3570002.png|600px|thumb|center|Fig. 2: CrtYB(ek)I-pUC19. The integrative locus used in yeast is HO. The selective locus used is URA3. The genes coding for CrtYB and CrtI come from <i>X. dendrorhous</i> and is fused using the artificial peptide linker ek. We use the TDH1 promoter and the tCYC1 terminator. ]] | |
| − | + | ||
| − | + | ||
| − | The | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
Revision as of 13:49, 20 October 2020
Provitamin A synthesis from GGPP in S. cerevisiae
Assembly Compatibility:
- 10INCOMPATIBLE WITH RFC[10]Illegal XbaI site found at 1297
Illegal PstI site found at 2357
Illegal PstI site found at 2627
Illegal PstI site found at 3605 - 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 2012
Illegal NheI site found at 5006
Illegal PstI site found at 2357
Illegal PstI site found at 2627
Illegal PstI site found at 3605 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 738
Illegal BglII site found at 3364
Illegal BglII site found at 5489
Illegal BglII site found at 6585
Illegal BamHI site found at 3157
Illegal XhoI site found at 4943
Illegal XhoI site found at 4984 - 23INCOMPATIBLE WITH RFC[23]Illegal XbaI site found at 1297
Illegal PstI site found at 2357
Illegal PstI site found at 2627
Illegal PstI site found at 3605 - 25INCOMPATIBLE WITH RFC[25]Illegal XbaI site found at 1297
Illegal PstI site found at 2357
Illegal PstI site found at 2627
Illegal PstI site found at 3605
Illegal NgoMIV site found at 2018 - 1000COMPATIBLE WITH RFC[1000]
Introduction
This biobrick shall be used to produce provitamin A (𝛽-carotene) in S. cerevisiae. 𝛽-carotene is one of the carotenoids produced in yeast. The metabolic pathway comprises multiple intermediate- as well as side-products before reaching 𝛽-carotene (fig. 1). It starts with geranylgeranyl diphosphate (GGPP), which is a derivative from Mevalonate pathway. GGPP is importantly used in yeast since it is a precursor to carotenoids[1], tocopherols[2], and to geranylgeranylated proteins[3]. Therefore, for the best production yield of 𝛽-carotene production using this biobrick, it is best to use it in synergy with "GGPP production enhancement in S. cerevisiae" biobrick (BBa_K3570000).
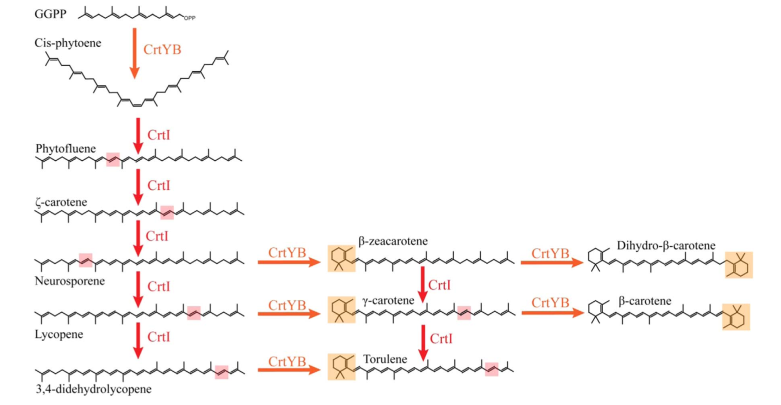
Fig. 1: From Rabeharindranto et al. 2019. Presumed biosynthetic pathway for the synthesis of β-carotene in X. dendrorhous (Verdoes et al., 1999). The bifunctional lycopene cyclase/phytoene synthase (CrtYB) enzyme is depicted in orange, the phytoene desaturase enzyme (CrtI) is depicted in red. Orange or red boxes represent the localization of the modifications introduced by the CrtYB or CrtI enzymes respectively.
Design
Experiments
Refernces
- [1]- Rabeharindranto, H., Castaño-Cerezo, S., Lautier, T., Garcia-Alles, L. F., Treitz, C., Tholey, A., & Truan, G. (2019). Enzyme-fusion strategies for redirecting and improving carotenoid synthesis in S. cerevisiae. Metabolic Engineering Communications, 8, e00086
- [2]- DIPLOCK, A. T., GREEN, J., EDWIN, E. E., & BUNYAN, J. (1961). Tocopherol, Ubiquinones and Ubichromenols in Yeasts and Mushrooms. Nature, 189(4766), 749–750. https://doi.org/10.1038/189749a0
- [3]- Ohya, Y., Qadota, H., Anraku, Y., Pringle, J. R., & Botstein, D. (1993). Suppression of yeast geranylgeranyl transferase I defect by alternative prenylation of two target GTPases, Rho1p and Cdc42p. Molecular Biology of the Cell, 4(10), 1017–1025. https://doi.org/10.1091/mbc.4.10.1017

