Difference between revisions of "Part:BBa K3304003"
PlatonMega (Talk | contribs) |
PlatonMega (Talk | contribs) |
||
| Line 7: | Line 7: | ||
https://2019.igem.org/wiki/images/d/d3/T--Thessaloniki--animation-step11.gif | https://2019.igem.org/wiki/images/d/d3/T--Thessaloniki--animation-step11.gif | ||
https://2019.igem.org/File:T--Thessaloniki--results-f1.png | https://2019.igem.org/File:T--Thessaloniki--results-f1.png | ||
| + | https://2019.igem.org/wiki/images/c/c1/T--Thessaloniki--results-f12.png | ||
| + | The bleaching of the reporter FAM observed throughout our reporter measurements. As the fluorophore becomes excited throughout the measurements it loses in fluorescence intensity resulting in a distinct dip observed in the graph. It is apparent that the more the fluorescence the more obvious the bleaching effect as more fluorophores are exposed | ||
https://2019.igem.org/wiki/images/9/97/T--Thessaloniki--results-f8.png | https://2019.igem.org/wiki/images/9/97/T--Thessaloniki--results-f8.png | ||
The voltage diagram from our MOSFET circuit that translates the binding of a single-stranded DNA to the bottom strand of the reporter complex that is probed to a DNA plated electrode. The binding is expressed through the change in capacitance and it is viewed as drop-in voltage readout. As a bigger concentration of the corresponding strand is bound to the DNA probes we observe a bigger dip in voltage | The voltage diagram from our MOSFET circuit that translates the binding of a single-stranded DNA to the bottom strand of the reporter complex that is probed to a DNA plated electrode. The binding is expressed through the change in capacitance and it is viewed as drop-in voltage readout. As a bigger concentration of the corresponding strand is bound to the DNA probes we observe a bigger dip in voltage | ||
Revision as of 03:00, 22 October 2019
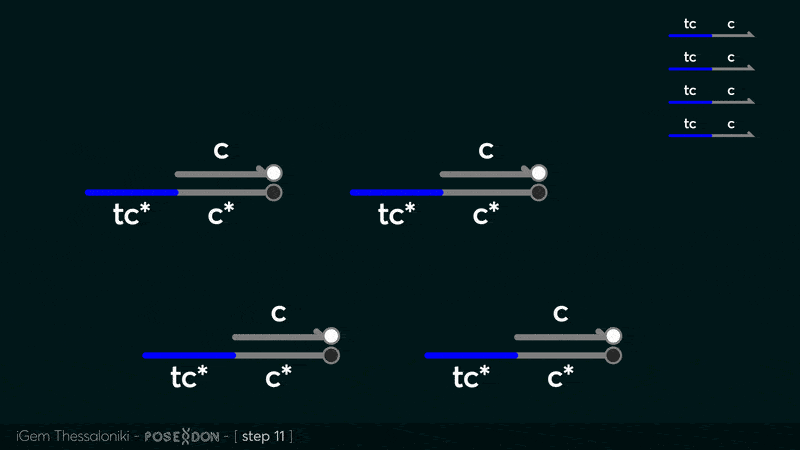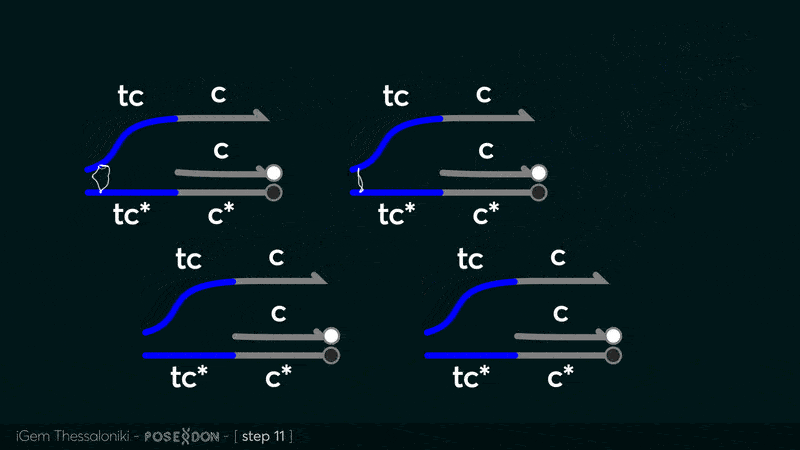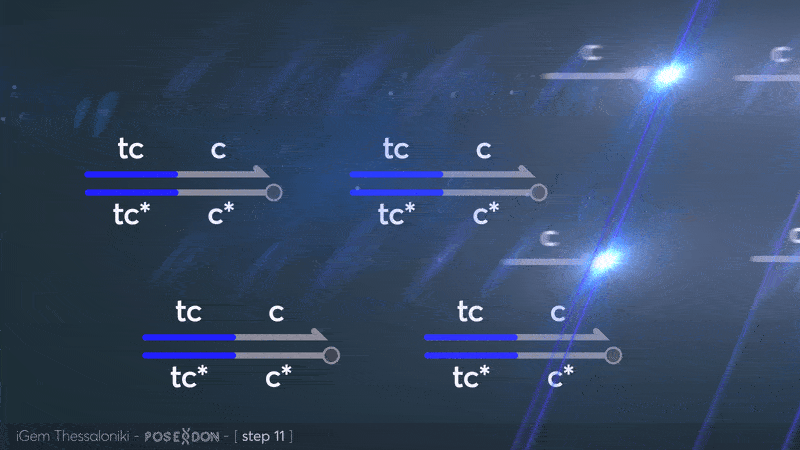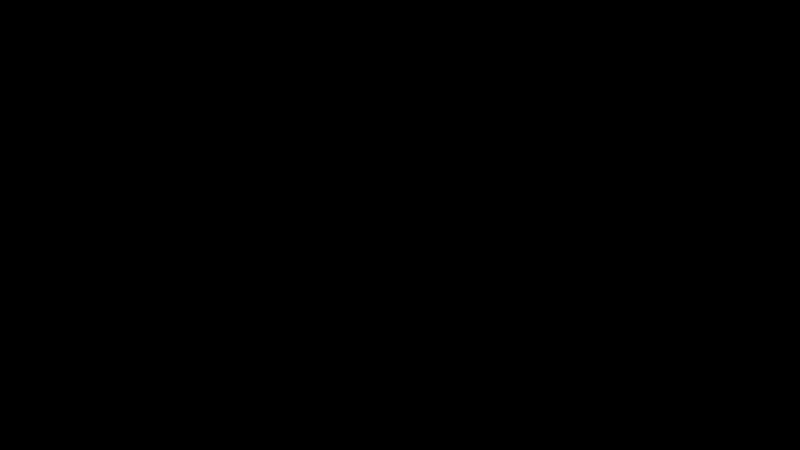
Reporter complex for output - TCC
This is the reporter complex that generates the fluorescent output to be measured as a quantitative readout of the formal CRN's function. The top strand is labeled with a fluorophore molecule at its 3' end while it's complementary strand is labeled with a quencher at it's 5' end. The quencher is able to absorb the fluorescence emitted by the fluorophore for as long as they're paired. The strand displacement reaction induces the separation of the quencher from the fluorophore producing measurable output.
 https://2019.igem.org/File:T--Thessaloniki--results-f1.png
https://2019.igem.org/File:T--Thessaloniki--results-f1.png
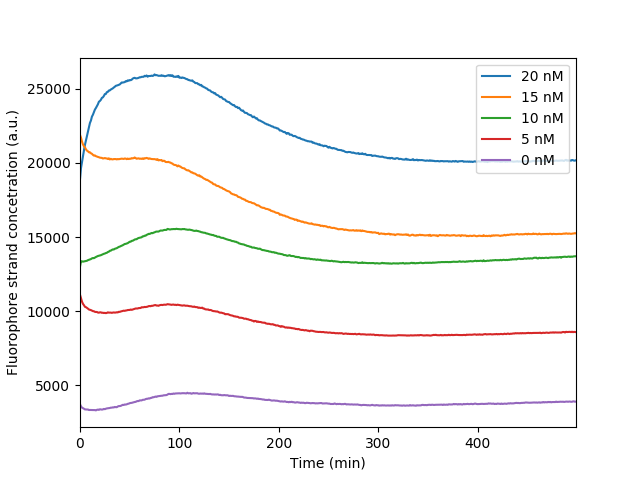 The bleaching of the reporter FAM observed throughout our reporter measurements. As the fluorophore becomes excited throughout the measurements it loses in fluorescence intensity resulting in a distinct dip observed in the graph. It is apparent that the more the fluorescence the more obvious the bleaching effect as more fluorophores are exposed
The bleaching of the reporter FAM observed throughout our reporter measurements. As the fluorophore becomes excited throughout the measurements it loses in fluorescence intensity resulting in a distinct dip observed in the graph. It is apparent that the more the fluorescence the more obvious the bleaching effect as more fluorophores are exposed
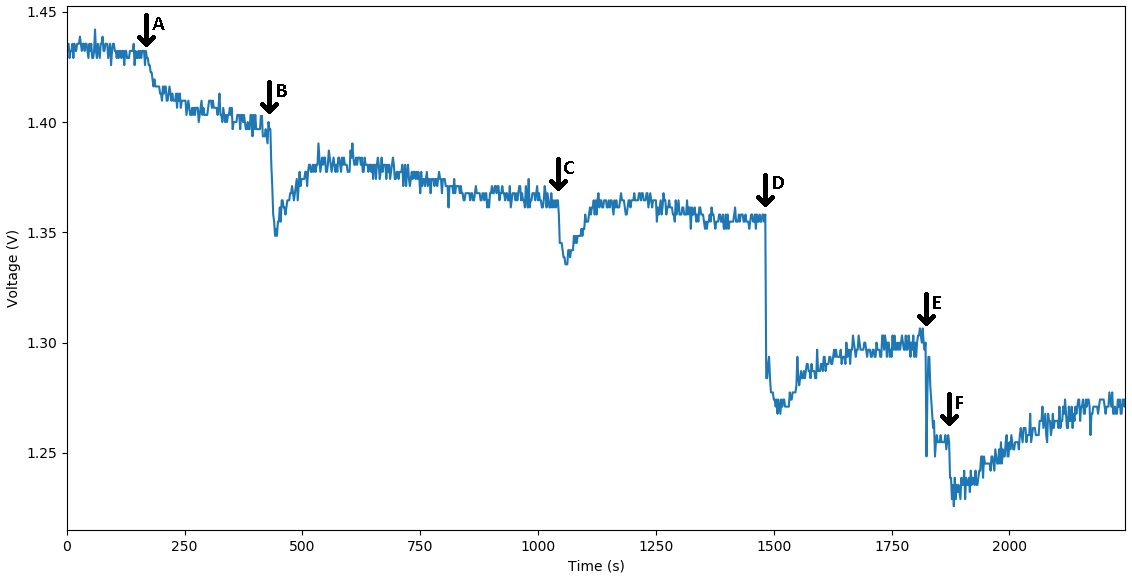 The voltage diagram from our MOSFET circuit that translates the binding of a single-stranded DNA to the bottom strand of the reporter complex that is probed to a DNA plated electrode. The binding is expressed through the change in capacitance and it is viewed as drop-in voltage readout. As a bigger concentration of the corresponding strand is bound to the DNA probes we observe a bigger dip in voltage
The voltage diagram from our MOSFET circuit that translates the binding of a single-stranded DNA to the bottom strand of the reporter complex that is probed to a DNA plated electrode. The binding is expressed through the change in capacitance and it is viewed as drop-in voltage readout. As a bigger concentration of the corresponding strand is bound to the DNA probes we observe a bigger dip in voltage
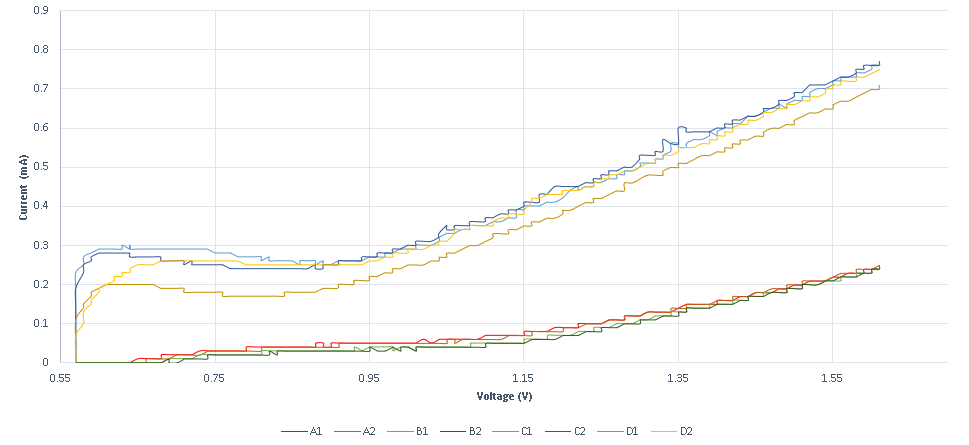
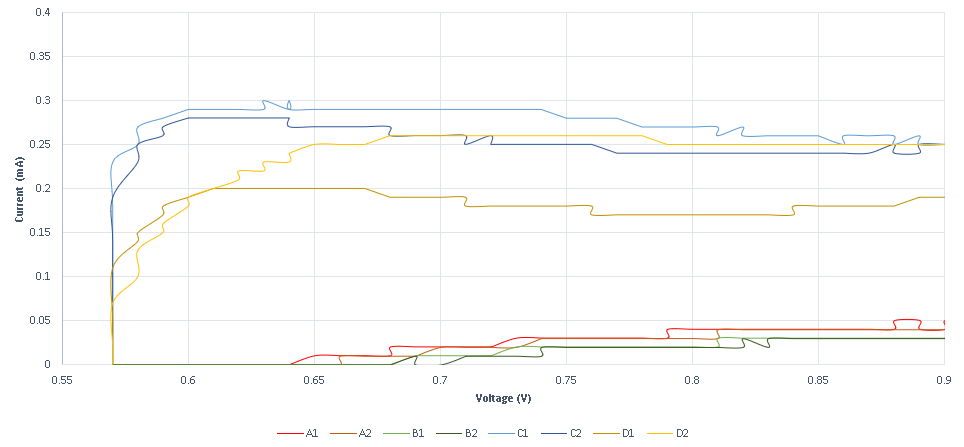 https://2019.igem.org/File:T--Thessaloniki--results-f1.png
https://parts.igem.org/File:BBa_K3304003-ReporterCharacterizationThessaloniki2019.png
https://2019.igem.org/File:T--Thessaloniki--results-f1.png
https://parts.igem.org/File:BBa_K3304003-ReporterCharacterizationThessaloniki2019.png
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
