Difference between revisions of "Part:BBa K3113100"
| Line 35: | Line 35: | ||
<figure class="figure"> | <figure class="figure"> | ||
<img src="https://2019.igem.org/wiki/images/e/e6/T--Munich--HiBiT_VLP_expression_and_export_coiled.png" width="auto" height="360" class="figure-img img-fluid rounded" alt="A generic square placeholder image with rounded corners in a figure."> | <img src="https://2019.igem.org/wiki/images/e/e6/T--Munich--HiBiT_VLP_expression_and_export_coiled.png" width="auto" height="360" class="figure-img img-fluid rounded" alt="A generic square placeholder image with rounded corners in a figure."> | ||
| − | <figcaption class="figure-caption"><b>Figure 2 | + | <figcaption class="figure-caption"><b>Figure 2: Gag levels in cells and in VLPs in the supernatant for the coiled-coil system.</b> The effect on the VLP-secretion efficiency of the Gag-HiBiT-P9SN protein was tested when interacting with the RBPs L7Ae and MCP through the P9SN/P10SN coiled-coil system. The HiBiT signal in the supernatant increases when P10SN-L7Ae is co-transfected with its target RNA-motif C/Dbox. The same applies to P10SN-MCP and the MS2 loop. Figure shows measurement of the HiBiT signal carried out for n = 8 biological replicates in a 96-well format. |
</figcaption> | </figcaption> | ||
Latest revision as of 02:34, 22 October 2019
Gag HIV-1
This sequence codes for the coat protein of the human immunodeficiency virus, which mediates the essential events in virion assembly, including binding the plasma membrane, making the protein-protein interactions necessary to create spherical particles.
Usage
For ALiVE, two vesicles were tested. One kind of vesicle is based on the Gag-p24 capsid protein of HIV. We used this protein fused together with RNA binding proteins to build and load virus-like particles for minimally-invasive cell monitoring.
Biologie
Gag-p24 is the group-specific antigen from the lentivirus HIV. It assembles at the plasma membrane and leads to the budding of vesicles.[1]
Characterization
Protein Structure

HiBiT
To furtherly prove the BioBrick Part we designed works as expected we performed a HiBiT split luciferase assay, which shows luminescent signal detected in formed VLPs. The following graph shows the amount of expressed Gag-HiBiT in cells compared to the amount measured in the supernatant. With this assay, we could show that 45 percent of Gag is exported to the supernatant in the form of vesicles.

Purification
Contrary to the exosomal protein CD63, the structural Gag protein forming the VLP shell contains no transmembrane domains and binds the vesicle membrane only on the cytoplasmic side. Therefore, it lacks extracellular regions that could be used to insert an affinity tag. We thus tested Heparin affinity chromatography, which has been reported previously (Reiter et al 2019) as a suitable method for Gag-based vesicle purification. As Figure 5 shows, we were able to robustly purify VLPs using Heparin chromatography. Overall, we could recover more than 50 % of VLPs with this purification method.

Dynamic Light Scattering
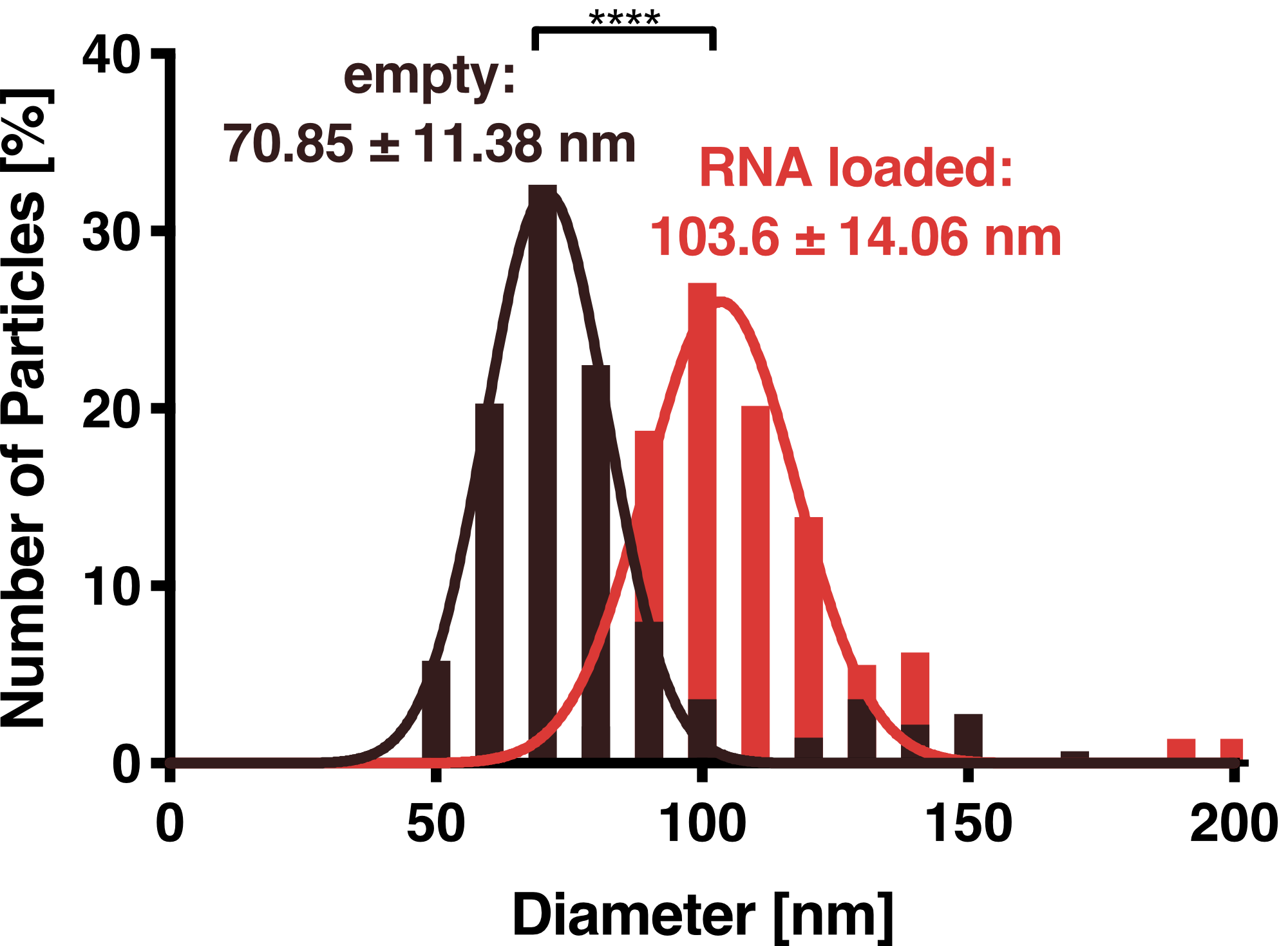
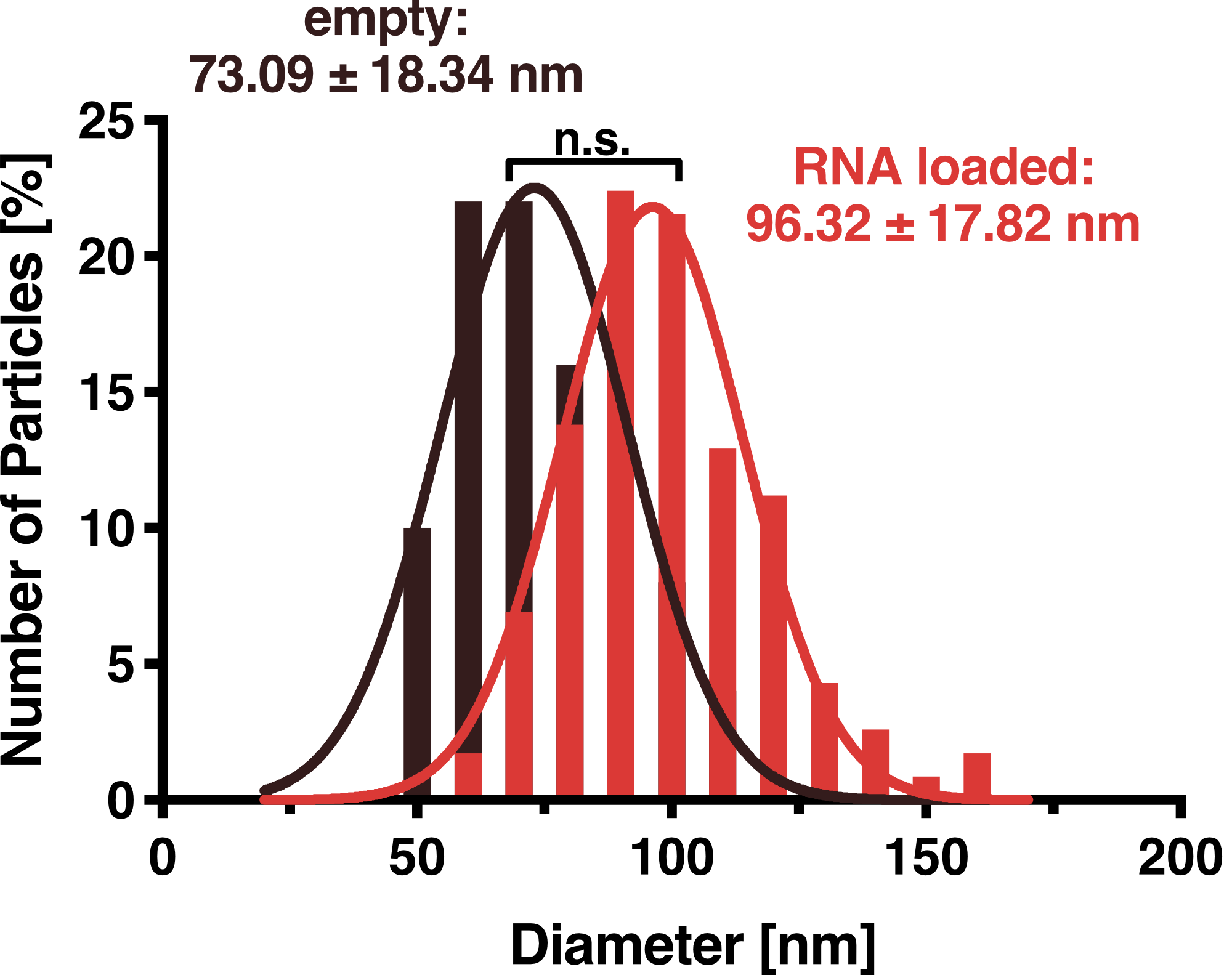
DLS measurements of VLP particles showed a narrow Gaussian size distribution indicating that the samples are very homogenous. Interestingly, a shift of about 30 nm is seen between cargo-loaded and unloaded VLPs; cargo-loaded particles have a mean diameter of 104 ± 14 nm, whereas unloaded vesicles showed a mean diameter of 71 ± 11 nm.
Transmission Electron Microscopy
To further validate that our vesicles are intact and properly shaped, we prepared purified VLPs and exosomes for transmission electron microscopy (TEM). This microscopy technique is based on a high-energy beam of electrons shown through a very thin sample fixed on a grid and allows high-resolution imaging.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1307
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 607
References
- ↑ HIV-1 Gag: a Molecular Machine Driving Viral Particle Assembly and Release Heinrich G. Göttlinger Department of Cancer Immunology and AIDS, Dana-Farber Cancer Institute, and Department of Pathology, Harvard Medical School, Boston
