Difference between revisions of "Part:BBa K3245001"
| Line 10: | Line 10: | ||
<h2>Characterization:</h2> | <h2>Characterization:</h2> | ||
<p>Measurement Protocol</p> | <p>Measurement Protocol</p> | ||
| − | <p>1. Transform a control plasmid containing luxI and luxR ( | + | <p>1. Transform a control plasmid containing luxI and luxR ( BBa_K3245002) with pUC ori ( high copy number ) and an effect plasmid containing luxpR-fus100 and GFP behind the promoter with p15A ori (medium copy number ) into DH10B. </p> |
<p>2. Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 3 ml LB with ampicillin at 100 μg/ml. Incubate overnight at 37℃ in a shaker.</p> | <p>2. Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 3 ml LB with ampicillin at 100 μg/ml. Incubate overnight at 37℃ in a shaker.</p> | ||
| − | <p>3. Measure and keep all groups OD600 reach 1. | + | <p>3. Measure and keep all groups OD600 reach 1. Inoculate each group with with 1/5000 concentration. Incubate 12 hours at 37℃ in a shaker.</p> |
<p>4. Add 100 µl bacteria culture medium into each well of a 96-well plate. One well of LB as blank, one group of wild type DH10B as control.</p> | <p>4. Add 100 µl bacteria culture medium into each well of a 96-well plate. One well of LB as blank, one group of wild type DH10B as control.</p> | ||
<p>5. Measure OD600 and fluorescence continuously every 30 minutes with a microplate reader.</p> | <p>5. Measure OD600 and fluorescence continuously every 30 minutes with a microplate reader.</p> | ||
[[File:T--Fudan--Part51.png|400px]] | [[File:T--Fudan--Part51.png|400px]] | ||
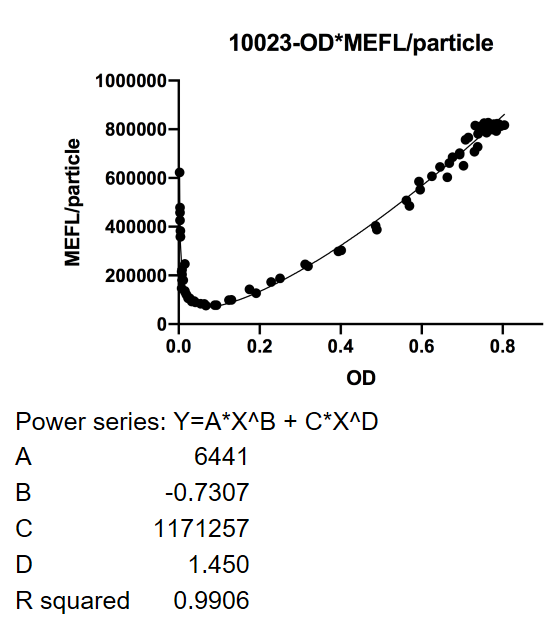
| + | <p><strong>Fig.1</strong> This figure shows luxpR-fus100 expression level induced by luxR-luxI box. The MEFL/particle changes as the OD goes up after dilution. | ||
Revision as of 18:36, 21 October 2019
luxpR-fus100:
This hybrid promoter has higher expression level when induced by luxR-AHL complex triggered by quorum sensing ( QS ) in some Gram-negative bacteria, meanwhile it’s leakage is also higher when not induced.
Usage and biology:
This fused promoter is designed for the situation that QS effect and high expression level are required at the same time in a circuit if leakage is acceptable to some extent. We applied this enhanced promoter to improve lacZ expression when bacteria reached a high density.
Design:
As the figure shows, we substituted the -35 to -10 region of the original promoter luxpR with the one of J23100, a strong constitutive promoter. This region is rarely concerned as it’s rather conservative in promoters with the same function, but it has crucial structural effect on σ factor binding and other events in transcription regulation. Fortunately the change proved to be effective on adjusting the behavior of the regulatory promoter.
Characterization:
Measurement Protocol
1. Transform a control plasmid containing luxI and luxR ( BBa_K3245002) with pUC ori ( high copy number ) and an effect plasmid containing luxpR-fus100 and GFP behind the promoter with p15A ori (medium copy number ) into DH10B.
2. Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 3 ml LB with ampicillin at 100 μg/ml. Incubate overnight at 37℃ in a shaker.
3. Measure and keep all groups OD600 reach 1. Inoculate each group with with 1/5000 concentration. Incubate 12 hours at 37℃ in a shaker.
4. Add 100 µl bacteria culture medium into each well of a 96-well plate. One well of LB as blank, one group of wild type DH10B as control.
5. Measure OD600 and fluorescence continuously every 30 minutes with a microplate reader.
Fig.1 This figure shows luxpR-fus100 expression level induced by luxR-luxI box. The MEFL/particle changes as the OD goes up after dilution.