Difference between revisions of "Part:BBa K3113302"
Theresakeil (Talk | contribs) |
Theresakeil (Talk | contribs) |
||
| Line 7: | Line 7: | ||
<h2>Usage</h2> | <h2>Usage</h2> | ||
| − | pCAG_GAG-HiBit-L7Ae construct is a Composite part that has been designed to achieve VLP formation inside HEK293T/MIN6-K8 cells and enable the transport of chosen RNA information inside vesicles through L7Ae-C/D box interaction (together with construct | + | pCAG_GAG-HiBit-L7Ae construct is a Composite part that has been designed to achieve VLP formation inside HEK293T/MIN6-K8 cells and enable the transport of chosen RNA information inside vesicles through L7Ae-C/D box interaction (<html>together with construct <a href="https://parts.igem.org/Part:BBa_K3113301">BBa_K3113301</a> and <a href="https://parts.igem.org/Part:BBa_K3113303">BBa_K3113303</ref> </html>). |
<h2>Biology</h2> | <h2>Biology</h2> | ||
Revision as of 14:12, 21 October 2019
pCAG_Gag-HiBiT-L7Ae
This plasmid codes for the coat protein of gag fused to a split luciferase fused to an RNA binding protein. This construct forms virus-like particles which can be detected via a split luciferase assay. Through the RNA binding protein, the vesicles can be loaded with RNA.
Usage
pCAG_GAG-HiBit-L7Ae construct is a Composite part that has been designed to achieve VLP formation inside HEK293T/MIN6-K8 cells and enable the transport of chosen RNA information inside vesicles through L7Ae-C/D box interaction (together with construct BBa_K3113301 and BBa_K3113303 ).
Biology
Gag-p24 is the group specific antigen from the lentivirus HIV. It assembles at the plasma membrane and leads to the budding of vesicles.[1]
“The Nano-Glo® HiBiT Lytic Detection System quantifies cellular protein in minutes with high sensitivity and a broad dynamic range using a simple add-mix-read assay format.”[2] The HiBiT peptide is an 11 amino acid long tag that can be added to the protein of interest. The tagged protein can be measured with the Nano-Glo® HiBiT Detection Reagent which contains the LbBiT. Together the HiBiT and the LgBiT form a functioning luminescent NanoBiT® enzyme.
The archaeal L7Ae and eukaryotic 15.5kD protein homologs are members of the L7Ae/15.5kD protein family that characteristically recognize K-turn motifs found in both archaeal and eukaryotic RNAs. In Archaea, the L7Ae protein uniquely binds the K-loop motif found in box C/D.[3]
The k-turn is a ubiquitous structural motif in RNA forming a very tight kink in the axis of helical RNA that plays an important role in many aspects of RNA function. L7Ae is a member of a superfamily of proteins that bind k-turns in RNA, stabilizing the tightly kinked conformation. They are extremely widespread and are important in the assembly of RNA–protein complexes central to translation, splicing and site-specific RNA modification. [4]
Characterization
Transmission Electron Microscopy (TEM)
Evidently, VLP formation from cells has been observed by transmission electron microscopy (TEM), proving the presence of the construct as the cause of VLP formation. GAG protein, encoded on the construct, is the main structural part of vesicle assembly.
Dynamic Light Scattering (DLS)
To confirm the size of VLPs calculated with the TEM pictures, we performed an analysis with dynamic light scattering.
Split-luciferase bioluminescent assay: The HiBiT Assay
To furtherly prove that the BioBrick Part we designed works as expected we performed a HiBiT split luciferase assay, which shows luminescent signal detected in formed VLPs.

Affinity Purification

Western Blot
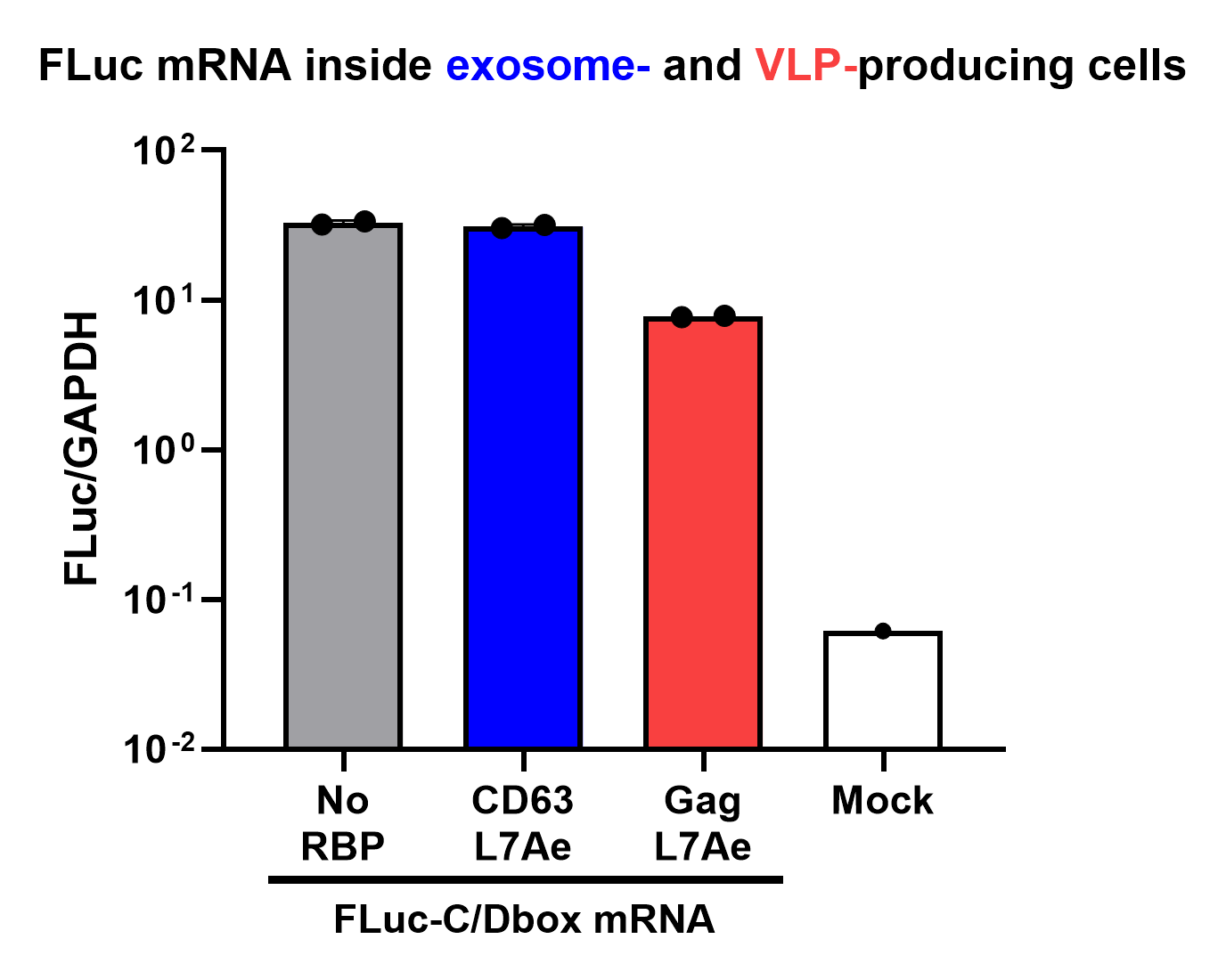
qPCR Analysis
Finally, the BioBrick Part we designed works as expected since chosen RNA information is successfully transported into VLPs through L7Ae-C/D box interaction. This has been proven through qPCR analysis of VLP content.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 3019
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 2319
References
- ↑ HIV-1 Gag: a Molecular Machine Driving Viral Particle Assembly and Release Heinrich G. Göttlinger Department of Cancer Immunology and AIDS, Dana-Farber Cancer Institute, and Department of Pathology, Harvard Medical School, Boston
- ↑ https://www.promega.de/products/protein-quantitation-and-detection/protein-quantitation/nano-glo-hibit-lytic-detection-system/?catNum=N3030
- ↑ Gagnon, Keith T et al. “Signature amino acids enable the archaeal L7Ae box C/D RNP core protein to recognize and bind the K-loop RNA motif.” RNA (New York, N.Y.) vol. 16,1 (2010): 79-90. doi:10.1261/rna.1692310
- ↑ Lilley, D. M. J. (2019). "The L7Ae proteins mediate a widespread and highly functional protein–RNA interaction." The Biochemist 41(2): 40-44.
