Difference between revisions of "Part:BBa K1720002"
Studentwang (Talk | contribs) |
Studentwang (Talk | contribs) |
||
| Line 7: | Line 7: | ||
==Experimental Results== | ==Experimental Results== | ||
[[File:T--LZU-CHINA--Hif(0).png|600px|thumb|center|]] | [[File:T--LZU-CHINA--Hif(0).png|600px|thumb|center|]] | ||
| + | ===Improved Results=== | ||
| + | *<b>Group:</b> LZU-CHINA 2019 | ||
| + | *<b>Author</b>: Jian Qi; Haodong Yan | ||
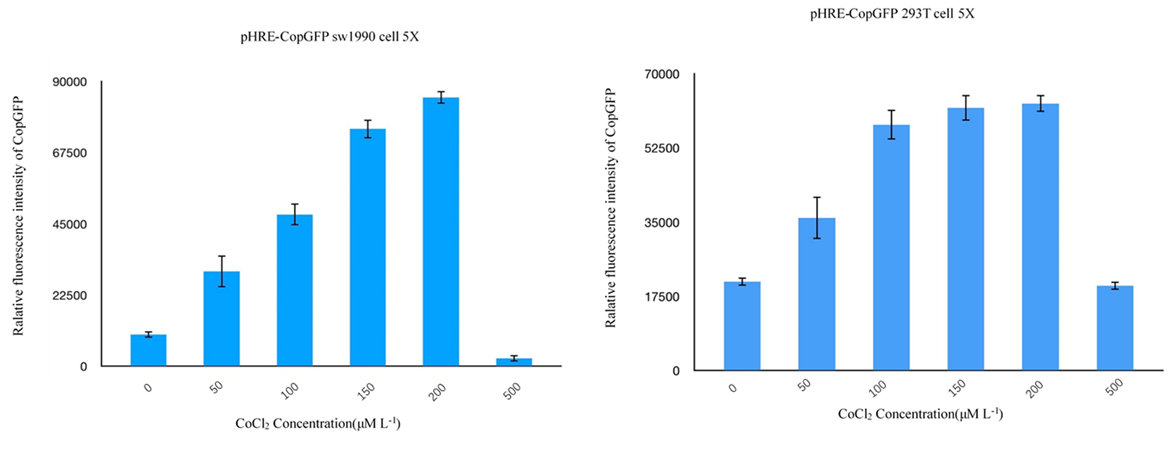
| + | *<b>Summary</b>: We modified a new HRE opereon with 5 reapts following miniCMV promoter(BBa_K1720002). We also had detected the function by monitoring the relative fluorescence intensity of copGFP with different CoCl(II) concentration. | ||
| + | [[File:T--LZU-CHINA--Hif(1).png|600px|thumb|center|]] | ||
| + | <ul> | ||
| + | <li>We tested the induced expression of pHRE with CopGFP. CoCl(II) is used to produce anoxic conditions. Figure 1~4 shows the effect of pHRE on HEK 293T cells. The experimental results show that the fluorescence intensity of CopGFP increases with the increase of CoCl(II) concentration. At a concentration of 500 uM, the fluorescence intensity decreased significantly. This is the result of inhibition of cell viability by high concentrations of CoCl(II). A similar effect was observed in SW1990 cells. In addition, the fluorescence intensity of SW1990 is usually lower than that of HEK293T, which may be due to the poor expression efficiency of SW1990 as a differentiated cell.</li> | ||
| + | </ul> | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
Revision as of 14:05, 21 October 2019
Hypoxia-induced promotor
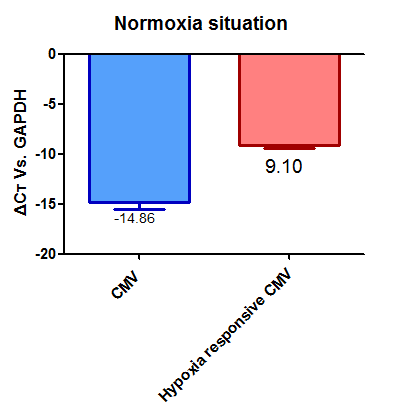
This part is a hypoxia response promotor.We used part BBa_K747096 as a backbone and inserted a hypoxia responsive element to the CMV promotor, so that the CMV promotor will work only under hypoxia situation. When the cells suffer from hypoxia situation this element will begin to work.It will activate the downstream gene expression.The hypoxia response element is a minimal cis-regulatory element mediating transactivation by the hypoxia-inducible factor (HIF) in mammalian cells.
This element with a GFP reporter was then transfected into HEK293 cells .The cells were either cultured under normoxia situation or treated with sodium hyposulfite, an oxygen cleaner to cause hypoxia situation, for 2 hours. As a control, HEK293 cells were also transiently transfected with carrying the original CMV promoter, submitted by the team Freiburg in 2012, followed by the EGFP reporter.
Experimental Results
Improved Results
- Group: LZU-CHINA 2019
- Author: Jian Qi; Haodong Yan
- Summary: We modified a new HRE opereon with 5 reapts following miniCMV promoter(BBa_K1720002). We also had detected the function by monitoring the relative fluorescence intensity of copGFP with different CoCl(II) concentration.
- We tested the induced expression of pHRE with CopGFP. CoCl(II) is used to produce anoxic conditions. Figure 1~4 shows the effect of pHRE on HEK 293T cells. The experimental results show that the fluorescence intensity of CopGFP increases with the increase of CoCl(II) concentration. At a concentration of 500 uM, the fluorescence intensity decreased significantly. This is the result of inhibition of cell viability by high concentrations of CoCl(II). A similar effect was observed in SW1990 cells. In addition, the fluorescence intensity of SW1990 is usually lower than that of HEK293T, which may be due to the poor expression efficiency of SW1990 as a differentiated cell.
Usage and Biology
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal SpeI site found at 41
- 12INCOMPATIBLE WITH RFC[12]Illegal SpeI site found at 41
- 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal SpeI site found at 41
- 25INCOMPATIBLE WITH RFC[25]Illegal SpeI site found at 41
- 1000COMPATIBLE WITH RFC[1000]
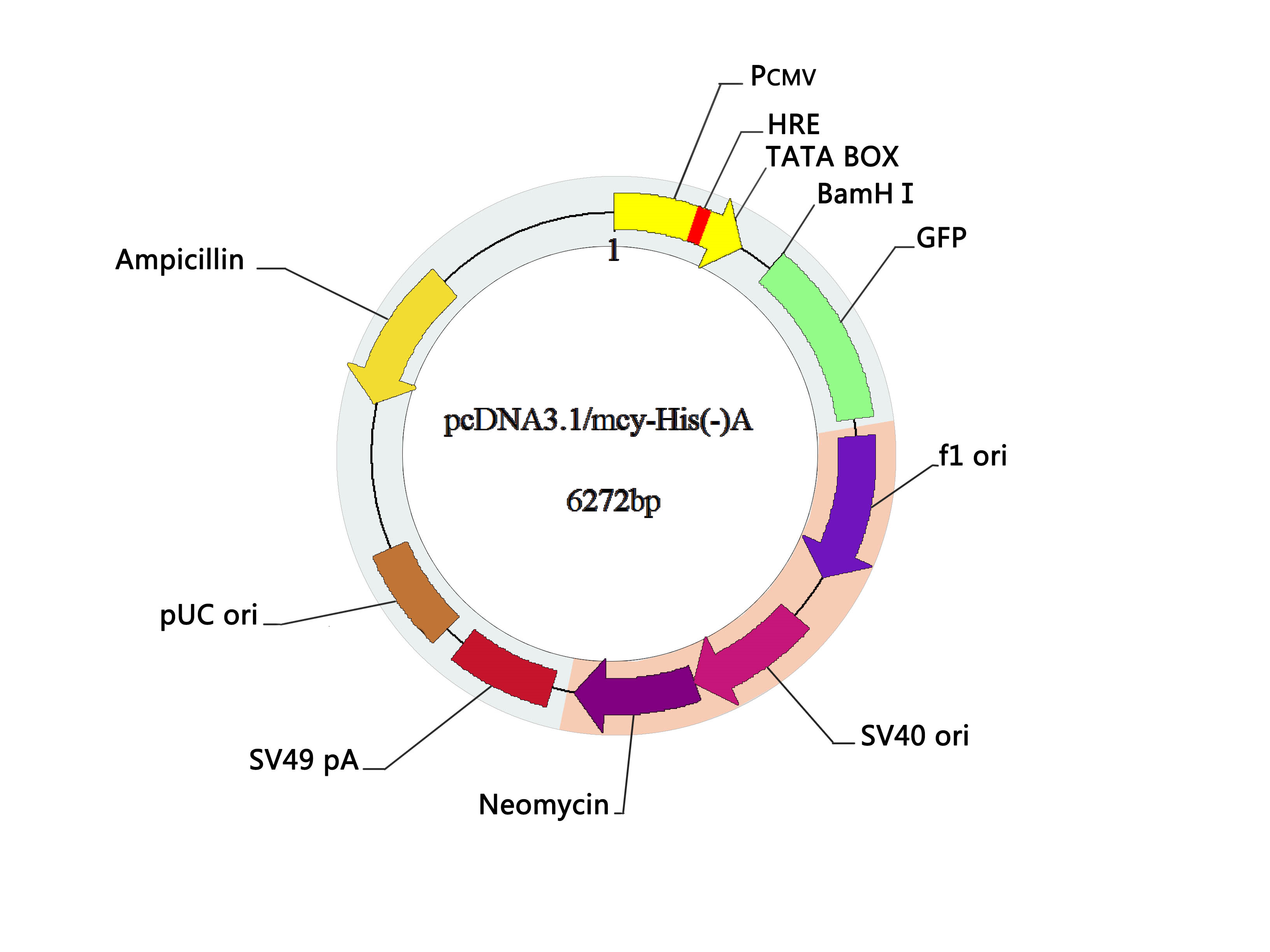
Before we start the experiment, we add our part to psb1c3 vector and packaged the vector with lipo2000.
Vector Map:
Experiment:
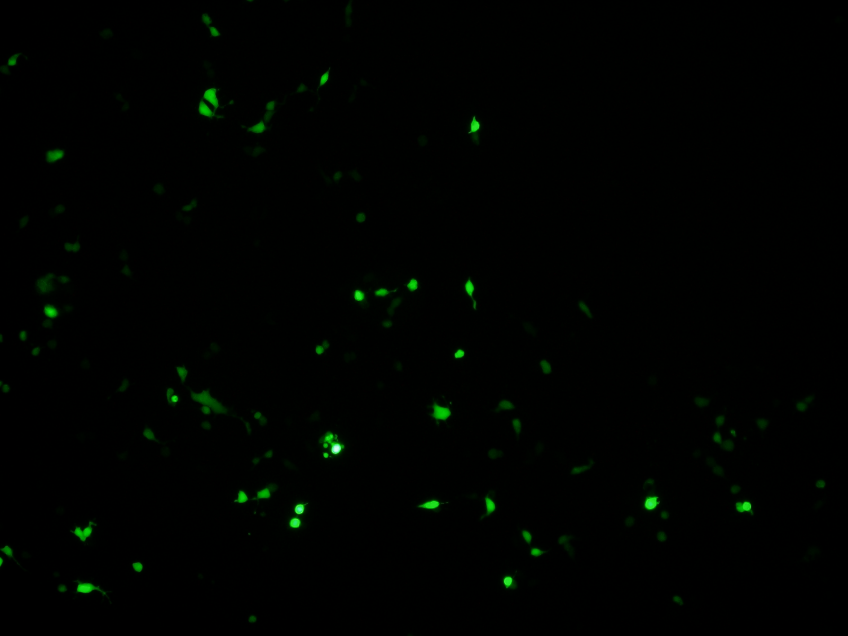
We transiently transfected HEK293 cells with plasmids containing hypoxia-induced promotor and EGFP reporter. The cells were treated with sodium hyposulfite, an oxygen cleaner to cause hypoxia situation.If green fluorescence signal was only observed in experimental group and positive control, our Hypoxia-induced promotor’s function will be demonstrated.
Protocol:
1. Seed cells to be 40% confluent at a 35mm culture dish.
2. Dilute 2.5ul Lipofectamine2000 Reagent in 50ul Opti-MEM Medium
3. Dilute 2.5ul (400ng/ul) plasmids in 50ul Opti-MEM Medium
4. Mix diluted Lipofectamine2000 Reagent with diluted plasmids, incubating for 5 min.
5. Withdraw culture medium from 35mm culture dish.
6. Add 1ml Opti-MEM Medium and plasmid-Lipo complex to cells
7. Incubate for 15 hours.
8. Withdraw medium from culture dish.
9. Add 2ml DMEM medium( containing 10% FBS )to cells and incubate for 10 hours
10. Withdraw culture medium from 35mm culture dish.
11. Add 20ul sodium hyposulfite(100umol/L ) to cells
12. Incubate for 1 hour.
13. Observe the cells under Inverted fluorescence microscope.
Result:
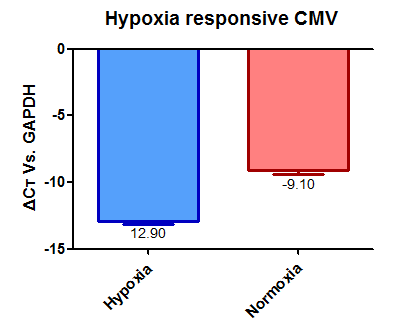
Under normoxia condition, we observed weaker green fluorescence under the control of HRE, the hypoxia responsive promotor, than that under the control of the original CMV promoter. Moreover, under the control of HRE, more cells exhibited green fluorescence under hypoxia condition than under normoxia condition. The results suggested that our HRE is working.
The overall florescent intensity under the control of HRE, however, were similar between hypoxia and normoxia conditions. After discussion, we thought there may be several issues in the model, i.e. slower growth of cells or weaker activity of EGFP under the hypoxia condition. Thus, we also measured expression of EGFP by real-time PCR. That data further proved that the HRE device worked as we expected, although not as strict. We believe we can improve this promotor and make it more sensitive in our future work.
References
BBa_K2295003 was sent in 2017 by Team Freiburg as an improvement of this BioBrick.