Difference between revisions of "Part:BBa K3016200"
| Line 3: | Line 3: | ||
<partinfo>BBa_K3016200 short</partinfo> | <partinfo>BBa_K3016200 short</partinfo> | ||
| − | + | This is a composite part containing <i>Vibrio natriegens'</i> TorA Tat signal peptide ([https://parts.igem.org/Part:BBa_K3016100 BBa_K3016100]) fused with YGFP ([https://parts.igem.org/Part:BBa_K3016600 BBa_K3016600]), a slow-bleaching GFP variant. | |
| + | |||
| + | TorA is a twin-arginine (RR) motif containing signal peptide for periplasmic transport of proteins via the twin-arginine translocation (Tat) pathway. Derived from Vibrio natriegens’ TMAO reductase (<i>torA</i>) gene. | ||
| + | |||
| + | Thus, the YGFP produced by this part should localise into the periplasm. | ||
| + | |||
| + | Note: TorA signal peptide may be prone to inclusion body formation in <i>Escherichia coli</i> (Jong <i>et al.</i>, 2017). Unconfirmed in <i>Vibrio natriegens</i>. | ||
| + | |||
| + | ===Biology=== | ||
| + | |||
| + | The twin-arginine translocation (Tat) pathway is capable of translocating fully folded proteins up to 150 kDa. It also contains a quality control feature of rejecting misfolded proteins. In some cases, disulfide bridge formation is not required for successful translocation. (Alanen et al., 2015) | ||
| + | |||
| + | Translocation using the tat-pathway requires the protein to contain a N-terminal signal peptide with a twin-arginine (RR) motif. The signal peptide is cleaved during the translocation process. A pair of <i>V. natriegens’</i> native twin-arginine signal peptides identified by Aalto-Helsinki can be found here ([https://parts.igem.org/Part:BBa_K3016100 TorA]and [https://parts.igem.org/Part:BBa_K3016100 Aminotransferase]) | ||
| + | |||
| + | ===Use=== | ||
| + | |||
| + | This part can be used to easily test protein localisation into the periplasm via the Tat pathway in <i>Vibrio natriegens</i>. | ||
| + | |||
| + | |||
| + | |||
| + | ==Characterization== | ||
| + | |||
| + | Aalto-Helsinki 2019 characterized this part by expressing it in <i>Vibrio natriegens</i> and <i>Escherichia coli</i> DH5a, testing its localisation into the periplasm. | ||
| + | |||
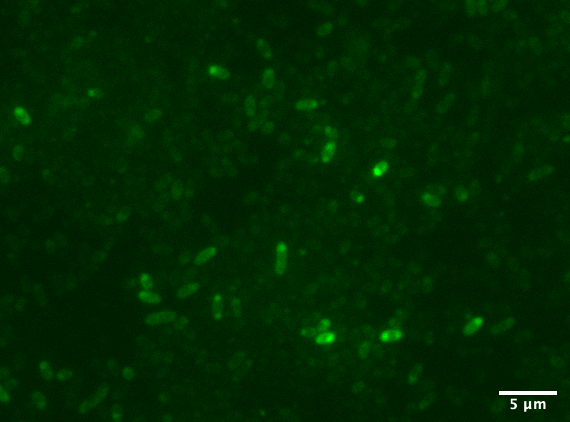
| + | [[Image:T--Aalto-Helsinki--TorA-YGFP_natriegens.png|thumb|530px|center|<font size="1">Vibrio natriegens cells expressing TorA-YGFP</font>]] | ||
| + | |||
| + | |||
| + | [[Image:T--Aalto-Helsinki--TorA-YGFP_coli.jpg|thumb|530px|center|<font size="1"><i>Escherichia coli</i> DH5a cells expressing TorA-YGFP</font>]] | ||
| + | |||
| + | |||
| + | In the images above we see <i>Vibrio natriegens</i> and <i>Escherichia coli</i> DH5a cells expressing TorA-YGFP. Note the polar localisation of fluorescence. This can be a sign of periplasmic localisation of YGFP under osmotic pressure (Sochacki <i>et al.</i>, 2011) or inclusion body formation (Jong <i>et al.</i>, 2017). | ||
| + | |||
| + | |||
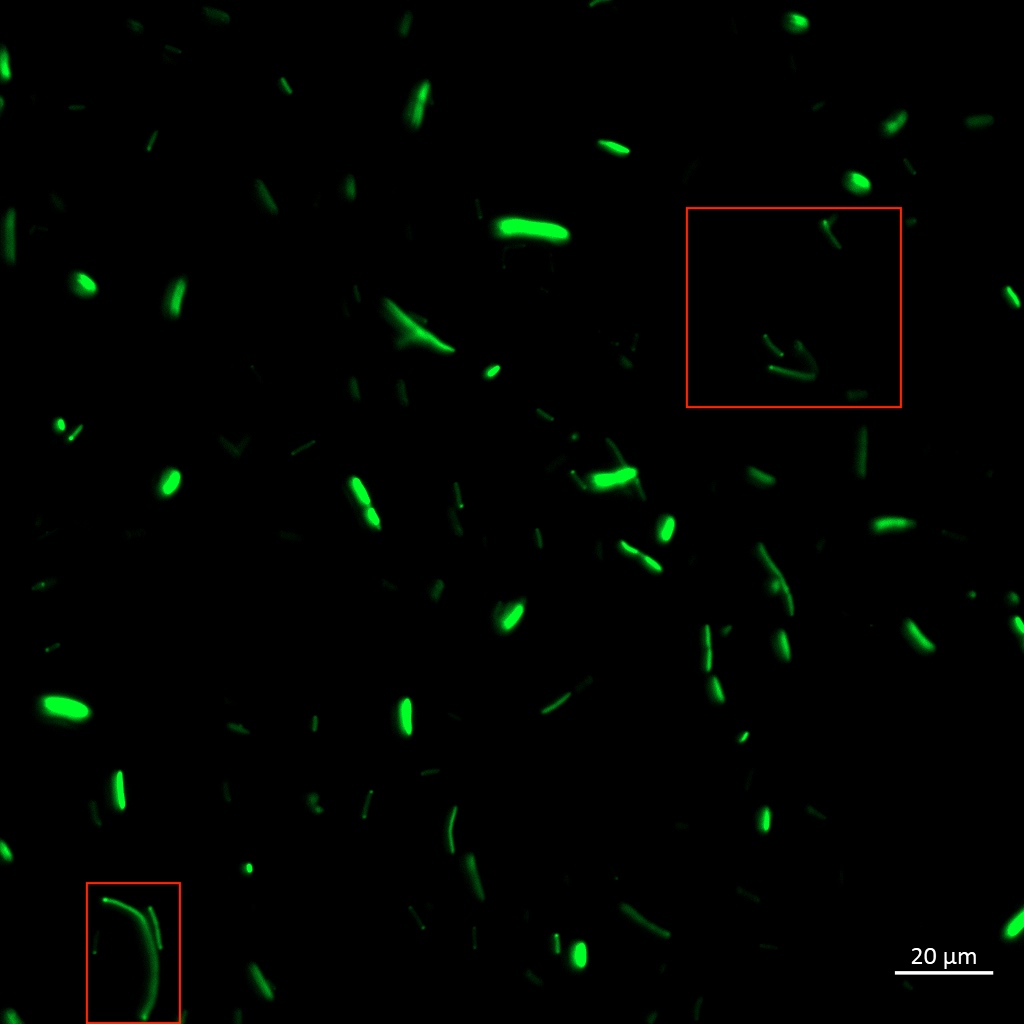
| + | To be certain of successful periplasmic translocation, a cell fractionation experiment was performed on <i>Vibrio natriegens</i>. The periplasmic fraction of TorA-YGFP expressing cells was extracted ([ protocol]) and placed under UV light, image below. The presence of YGFP in the periplasmic fractions can be seen clearly. | ||
| + | |||
| + | |||
| + | [[Image:T--Aalto-Helsinki--TorA-YGFP_natriegens_periplasmic_fraction.jpg|thumb|530px|center|<font size="1">Periplasmic fractions of Vibrio natriegens cells expressing TorA-YGFP </font>]] | ||
| + | |||
| + | |||
| + | These results indicate that the <i>Vibrio natriegens'</i> TorA-YGFP successfully translocates into the periplasm of <i>Vibrio natriegens</i>, and possibly even of <i>Escherichia coli</i>. | ||
| − | |||
| − | |||
| − | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K3016200 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3016200 SequenceAndFeatures</partinfo> | ||
| + | |||
| + | ==References:== | ||
| + | |||
| + | Alanen, H. I., Walker, K. L., Suberbie, M. L. V., Matos, C. F., Bönisch, S., Freedman, R. B., ... & Robinson, C. (2015). Efficient export of human growth hormone, interferon α2b and antibody fragments to the periplasm by the Escherichia coli Tat pathway in the absence of prior disulfide bond formation. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1853(3), 756-763. | ||
| + | |||
| + | Jong, W. S., Vikström, D., Houben, D., de Gier, J. W., & Luirink, J. (2017). Application of an E. coli signal sequence as a versatile inclusion body tag. Microbial cell factories, 16(1), 50. | ||
| + | |||
| + | Sochacki, K. A., Shkel, I. A., Record, M. T., & Weisshaar, J. C. (2011). Protein diffusion in the periplasm of E. coli under osmotic stress. Biophysical journal, 100(1), 22-31. | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Revision as of 10:29, 21 October 2019
Vibrio natriegens' TorA signal peptide containing YGFP gene
This is a composite part containing Vibrio natriegens' TorA Tat signal peptide (BBa_K3016100) fused with YGFP (BBa_K3016600), a slow-bleaching GFP variant.
TorA is a twin-arginine (RR) motif containing signal peptide for periplasmic transport of proteins via the twin-arginine translocation (Tat) pathway. Derived from Vibrio natriegens’ TMAO reductase (torA) gene.
Thus, the YGFP produced by this part should localise into the periplasm.
Note: TorA signal peptide may be prone to inclusion body formation in Escherichia coli (Jong et al., 2017). Unconfirmed in Vibrio natriegens.
Biology
The twin-arginine translocation (Tat) pathway is capable of translocating fully folded proteins up to 150 kDa. It also contains a quality control feature of rejecting misfolded proteins. In some cases, disulfide bridge formation is not required for successful translocation. (Alanen et al., 2015)
Translocation using the tat-pathway requires the protein to contain a N-terminal signal peptide with a twin-arginine (RR) motif. The signal peptide is cleaved during the translocation process. A pair of V. natriegens’ native twin-arginine signal peptides identified by Aalto-Helsinki can be found here (TorAand Aminotransferase)
Use
This part can be used to easily test protein localisation into the periplasm via the Tat pathway in Vibrio natriegens.
Characterization
Aalto-Helsinki 2019 characterized this part by expressing it in Vibrio natriegens and Escherichia coli DH5a, testing its localisation into the periplasm.
In the images above we see Vibrio natriegens and Escherichia coli DH5a cells expressing TorA-YGFP. Note the polar localisation of fluorescence. This can be a sign of periplasmic localisation of YGFP under osmotic pressure (Sochacki et al., 2011) or inclusion body formation (Jong et al., 2017).
To be certain of successful periplasmic translocation, a cell fractionation experiment was performed on Vibrio natriegens. The periplasmic fraction of TorA-YGFP expressing cells was extracted ([ protocol]) and placed under UV light, image below. The presence of YGFP in the periplasmic fractions can be seen clearly.
These results indicate that the Vibrio natriegens' TorA-YGFP successfully translocates into the periplasm of Vibrio natriegens, and possibly even of Escherichia coli.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 781
- 1000COMPATIBLE WITH RFC[1000]
References:
Alanen, H. I., Walker, K. L., Suberbie, M. L. V., Matos, C. F., Bönisch, S., Freedman, R. B., ... & Robinson, C. (2015). Efficient export of human growth hormone, interferon α2b and antibody fragments to the periplasm by the Escherichia coli Tat pathway in the absence of prior disulfide bond formation. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1853(3), 756-763.
Jong, W. S., Vikström, D., Houben, D., de Gier, J. W., & Luirink, J. (2017). Application of an E. coli signal sequence as a versatile inclusion body tag. Microbial cell factories, 16(1), 50.
Sochacki, K. A., Shkel, I. A., Record, M. T., & Weisshaar, J. C. (2011). Protein diffusion in the periplasm of E. coli under osmotic stress. Biophysical journal, 100(1), 22-31.