Difference between revisions of "Part:BBa K3260036"
Maryeliz46 (Talk | contribs) |
|||
| Line 1: | Line 1: | ||
| + | __NOTOC__ | ||
| + | <partinfo>BBa_K3260036 short</partinfo> | ||
| + | This part is a composite of the ZR leucine zipper (BBa_K3260029), cGFP (BBa_K3260028), and Imperial 2014’s dCBD (BBa_K1321340), all fused via flex linkers, or BBa_K3260026). It acts in conjunction with BBa_K3260034 to create a cellulose stabilized complex presents a hydrophobic head and also fluoresces upon successful dimerization. | ||
| + | |||
| + | |||
| + | ===Usage and Biology:=== | ||
| + | <br> | ||
| + | The design of two parts takes the burden off of a single construct to contain all three functional domains we wanted to express in our project (hydrophobic domain, fluorescent domain and cellulose binding domain). | ||
| + | We achieved this by splitting GFP into C and N domains (BBa_K3260028 and BBa_K3260030, respectively), and fused one part of a complementary leucine zipper pair with each GFP half. We then attached a dCBD to one part, and a hydrophobic domain to the other, and we had our two composite parts and a functional heterodimer. This part in particular took the burden of the dCBD, the cGFP half, and the ZR leucine zipper. The leucine zipper pair is the main actor when it comes to initiating proximity between these two complementary composite parts, and the function of BBa_K3260036 is verified and explained below. | ||
| + | |||
| + | https://2019.igem.org/wiki/images/f/f9/T--BrownStanfordPrinctn--3634_fluoresence_testing.png | ||
| + | https://2019.igem.org/wiki/images/a/a6/T--BrownStanfordPrinctn--3634_fluoresence_swatches.png | ||
| + | |||
| + | |||
| + | Above is a test of the functionality of two of our new fusion proteins, BBa_K3260036, and BBa_K3260034. If these two parts assemble correctly on the cellulose surface, there should be fluorescence, which is what we observed here. This means that the leucine zipper components of each protein aligned and held together, allowing the two halves of GFP to get close enough together to complete their chromophore and fluoresce. Because we see fluorescence (highlighted in the color swatch of each sample), the success of the leucine zippers and therefore the functionality of the last two functional domains can be inferred as well, the double cellulose binding domain anchoring the whole complex to the cellulose surface, as well as the hydrophobic domain sticking up from the N-terminal region of BBa_K3260034, and therefore the assembled protein complex. The successful assembly of these two biobricks not only shows the applicability of these constructs for paper-based microfluidics, but it also shows the potential of split-GFP reporting for fusion proteins in functional applications. | ||
| + | |||
| + | |||
| + | |||
| + | https://2019.igem.org/wiki/images/8/82/T--BrownStanfordPrinctn--3634_assembly_diagram.png | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <!-- --> | ||
| + | <span class='h3bb'>Sequence and Features</span> | ||
| + | <partinfo>BBa_K3260036 SequenceAndFeatures</partinfo> | ||
| + | |||
| + | |||
| + | <!-- Uncomment this to enable Functional Parameter display | ||
| + | ===Functional Parameters=== | ||
| + | <partinfo>BBa_K3260036 parameters</partinfo> | ||
| + | <!-- --> | ||
Revision as of 07:11, 21 October 2019
ZR + cGFP + dCBD
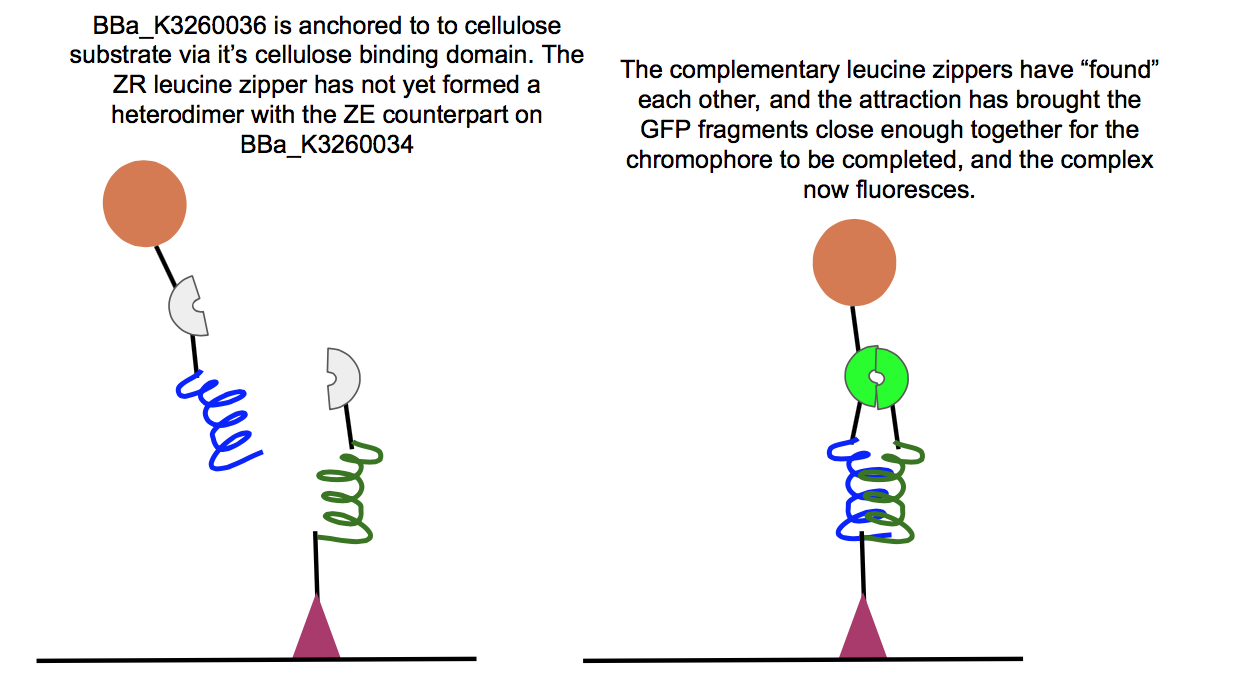
This part is a composite of the ZR leucine zipper (BBa_K3260029), cGFP (BBa_K3260028), and Imperial 2014’s dCBD (BBa_K1321340), all fused via flex linkers, or BBa_K3260026). It acts in conjunction with BBa_K3260034 to create a cellulose stabilized complex presents a hydrophobic head and also fluoresces upon successful dimerization.
Usage and Biology:
The design of two parts takes the burden off of a single construct to contain all three functional domains we wanted to express in our project (hydrophobic domain, fluorescent domain and cellulose binding domain).
We achieved this by splitting GFP into C and N domains (BBa_K3260028 and BBa_K3260030, respectively), and fused one part of a complementary leucine zipper pair with each GFP half. We then attached a dCBD to one part, and a hydrophobic domain to the other, and we had our two composite parts and a functional heterodimer. This part in particular took the burden of the dCBD, the cGFP half, and the ZR leucine zipper. The leucine zipper pair is the main actor when it comes to initiating proximity between these two complementary composite parts, and the function of BBa_K3260036 is verified and explained below.


Above is a test of the functionality of two of our new fusion proteins, BBa_K3260036, and BBa_K3260034. If these two parts assemble correctly on the cellulose surface, there should be fluorescence, which is what we observed here. This means that the leucine zipper components of each protein aligned and held together, allowing the two halves of GFP to get close enough together to complete their chromophore and fluoresce. Because we see fluorescence (highlighted in the color swatch of each sample), the success of the leucine zippers and therefore the functionality of the last two functional domains can be inferred as well, the double cellulose binding domain anchoring the whole complex to the cellulose surface, as well as the hydrophobic domain sticking up from the N-terminal region of BBa_K3260034, and therefore the assembled protein complex. The successful assembly of these two biobricks not only shows the applicability of these constructs for paper-based microfluidics, but it also shows the potential of split-GFP reporting for fusion proteins in functional applications.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
