Difference between revisions of "Part:BBa K1696005"
Ggjyliuyue (Talk | contribs) (→Reference) |
|||
| Line 24: | Line 24: | ||
<partinfo>BBa_K1696005 parameters</partinfo> | <partinfo>BBa_K1696005 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | ==2019-SZPT-CHINA== | ||
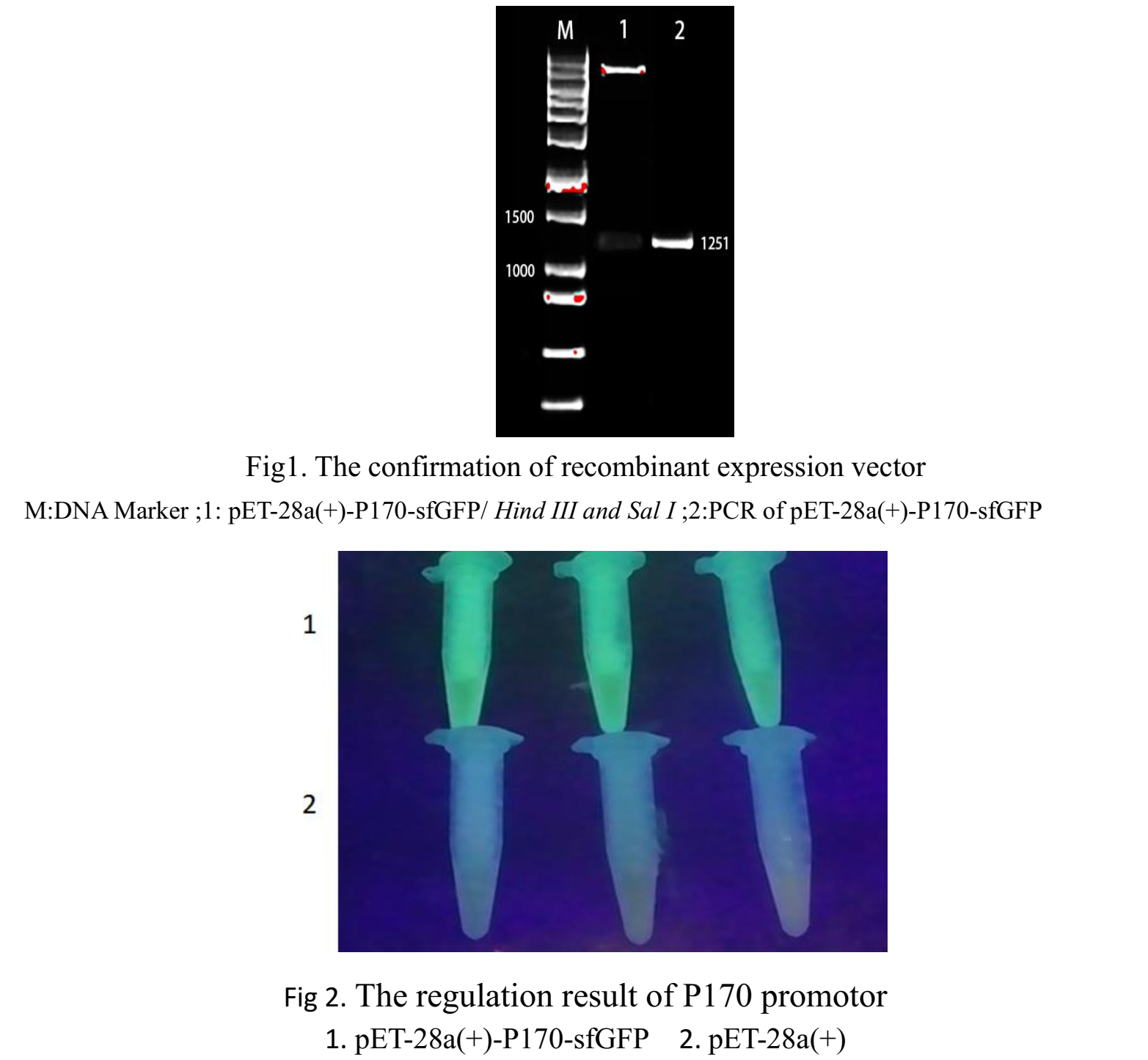
| + | First, we inserted P170-sfGFP the ''E. coli'' expression vector pET-28a(+). Fig.1 showed that the P170-sfGFP with size 1251bp was cloned to pET-28a(+) vector successfully. | ||
| + | [[File:P170.png|center|500px]] | ||
| + | Then the recombinant vector pET-28a(+)-P170-sfGFP was transformed into ''E. coli BL21''. The recombinant strain ''BL21'' with pET-28a(+)-P170-sfGFP was selected and inoculated in LB medium with pH 5. ''BL21'' with pET-28a(+) was used as control. After Incubate for 18 h at 37 ° C. The bacterium was placed under UV light to excite sfGFP. The results are shown in the Fig.2. The green sfGFP from ''BL21'' transferred with pET-28a(+)-P170-sfGFP of sfGFP be seen obviously in pH 5. | ||
| + | ''BL21'' transferred with pET-28a(+)-P170-sfGFP and ''BL21'' transferred with pET-28a(+) were inoculated in LB medium with pH 5 and pH 7 respectively. we measured the Fluorescence intensity using fluorescence intensity detection microplate reader. The result is showed in Fig.3.The result showed that the fluorescence intensity of ''BL21'' with pET-28a(+)-P170-sfGFP t was much higher than control ''BL21''. In pH 5, the fluorescence intensity using prompter P170 is 123.693. while in pH7, the fluorescence intensity using P170 is 101.407. | ||
| + | [[File:P170 1.png|center|500px]] | ||
Revision as of 08:35, 20 October 2019
P170 Promoter
P170 is upregulated at low pH (6.0~6.5) during the transition to stationary phase. The minimal DNA region required for both promoter activity and pH regulation was mapped to a 51 bp fragment located 7 bp upstream of the transcriptional start site. This fragment lacked the consensus -35 promoter region, but it contained an ‘extended’ -10 promoter region[1]. Besides, a novel 14 bp regulatory DNA region centered at around -41.5 and composed of three tetra nucleotide sequences, boxes A, C and D. Boxes A and C contribute to P170 activity, whereas box D and the position of boxes ACD (renamed ACiD-box) are essential to P170 activity and acid response[2].
Reference
[1] Madsen S M, Arnau J, Vrang A, et al. Molecular characterization of the pH-inducible and growth phase-dependent promoter P170 of Lactococcus lactis.[J]. Molecular Microbiology, 1999, 32(1):75-87.
[2] Søren M. Madsen, Thomas Hindré, Jean-Paul Le Pennec, et al. Two acid-inducible promoters from Lactococcus lactis require the cis-acting ACiD-box and the transcription regulator RcfB[J]. Molecular Microbiology, 2005, 56(3):735-746.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
2019-SZPT-CHINA
First, we inserted P170-sfGFP the E. coli expression vector pET-28a(+). Fig.1 showed that the P170-sfGFP with size 1251bp was cloned to pET-28a(+) vector successfully.
Then the recombinant vector pET-28a(+)-P170-sfGFP was transformed into E. coli BL21. The recombinant strain BL21 with pET-28a(+)-P170-sfGFP was selected and inoculated in LB medium with pH 5. BL21 with pET-28a(+) was used as control. After Incubate for 18 h at 37 ° C. The bacterium was placed under UV light to excite sfGFP. The results are shown in the Fig.2. The green sfGFP from BL21 transferred with pET-28a(+)-P170-sfGFP of sfGFP be seen obviously in pH 5. BL21 transferred with pET-28a(+)-P170-sfGFP and BL21 transferred with pET-28a(+) were inoculated in LB medium with pH 5 and pH 7 respectively. we measured the Fluorescence intensity using fluorescence intensity detection microplate reader. The result is showed in Fig.3.The result showed that the fluorescence intensity of BL21 with pET-28a(+)-P170-sfGFP t was much higher than control BL21. In pH 5, the fluorescence intensity using prompter P170 is 123.693. while in pH7, the fluorescence intensity using P170 is 101.407. center|500px