Difference between revisions of "Part:BBa I719005"
(→IV References) |
(→I Overview) |
||
| Line 43: | Line 43: | ||
=ZJU-China 2019's improvement= | =ZJU-China 2019's improvement= | ||
==I Overview== | ==I Overview== | ||
| − | This year team ZJU-China has upgraded four modified T7 promoters with increased strength (<partinfo>K2965011</partinfo>,<partinfo>K2965012</partinfo>,<partinfo>K2965013</partinfo>,<partinfo>K2965014</partinfo>). | + | This year team ZJU-China has upgraded four modified T7 promoters with increased strength (<partinfo>K2965011</partinfo>, <partinfo>K2965012</partinfo>, <partinfo>K2965013</partinfo>, <partinfo>K2965014</partinfo>). |
With the help of previous research, we carefully chose the site which would be mutated by PCR. These sites distribute in the range from -3 to +6. We mutated these sites by adding variable bases to primers (taatacgactcactatagggaga → taatacgactcacWNNNgSRRNN), and screened for stronger mutants. | With the help of previous research, we carefully chose the site which would be mutated by PCR. These sites distribute in the range from -3 to +6. We mutated these sites by adding variable bases to primers (taatacgactcactatagggaga → taatacgactcacWNNNgSRRNN), and screened for stronger mutants. | ||
For details, please refer to https://2019.igem.org/Team:ZJU-China/Improve. | For details, please refer to https://2019.igem.org/Team:ZJU-China/Improve. | ||
Revision as of 15:37, 19 October 2019
T7 Promoter
Constitutive promoter derived from the T7 bacteriophage. Allows high expression of proteins only when the T7 polymerase is present. This part is identical to the part BBa_R0085 which currently hasn't been built.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
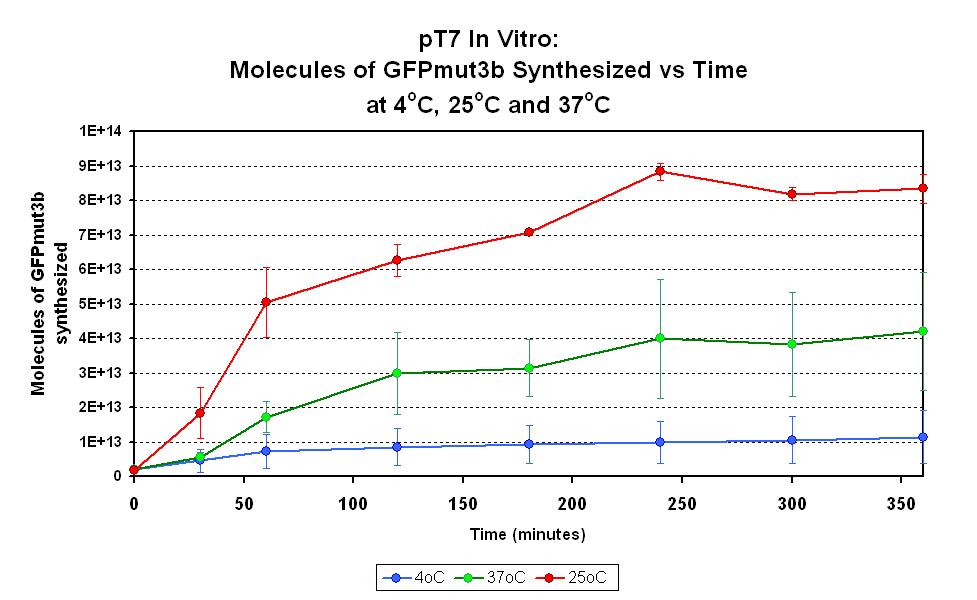
Characterization in vitro
This part has been characterized for temperature sensitivity in the Cell-Free Chassis by iGEM Imperial 2007. For more detail, please check the testing [http://2007.igem.org/Imperial/Wet_Lab/Protocols/Prot1.9 protocols]and [http://2007.igem.org/Imperial/Wet_Lab/Results/Res1.9 results].
| Parameter | Value and Description |
|---|---|
| Optimum temperature | The T7 promoter has a highest output at 25°C, with a one-fold increase in GFP molecules synthesized compared to 37°C. The T7 promoter also has a minimal amount of output at 4°C. |
| Expression Life-span | The rate of GFP synthesis by the T7 promoter reaches a peak at around 30-60 minutes. |
| Peak Expression | The production of GFP decreases to minimal levels after 2 hours and tends towards nil after 4 hours. |
Team Warsaw 2010's measurement
Absolute promoter strength: 41,8pg RNA/minute/ug substrate DNA. It equals 8,92 microPoPSContribution
Group: Valencia_UPV iGEM 2018
Author: Adrián Requena Gutiérrez, Carolina Ropero
Summary: We adapted the part to be able to assemble transcriptional units with the Golden Gate assembly method
Documentation:
In order to create our complete [http://2018.igem.org/Team:Valencia_UPV/Part_Collection part collection] of parts compatible with the Golden Gate assembly method, we made the part BBa_K2656000 which is this part adapted to the Golden Gate technology.
ZJU-China 2019's improvement
I Overview
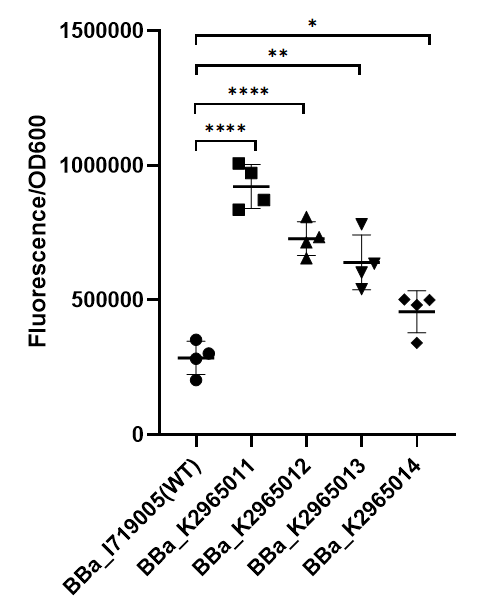
This year team ZJU-China has upgraded four modified T7 promoters with increased strength (BBa_K2965011, BBa_K2965012, BBa_K2965013, BBa_K2965014). With the help of previous research, we carefully chose the site which would be mutated by PCR. These sites distribute in the range from -3 to +6. We mutated these sites by adding variable bases to primers (taatacgactcactatagggaga → taatacgactcacWNNNgSRRNN), and screened for stronger mutants. For details, please refer to https://2019.igem.org/Team:ZJU-China/Improve.
II Result
To test the function of mutant promoters, we chose eGFP as our reporter, and added a lac operator behind the promotor to control transcription starts simultaneously. When the E.coli BL21(DE3) is cultured at the stage of logarithmic phase, we added 0.5 mM IPTG to induce the expression of GFP in strains BL21(DE3) for 4 hours. By assessing the absolute fluorescence and OD600, we can conclude the relative strength of all promoters. We screened out four mutants with higher intensity.
As we can see from the figure, our mutant promoters showed largely increased strength compared with wild type T7 promoter. Therefore, our mutant promoters offer users more opportunity to control the expression of protein using T7 promoter and permit higher levels of target protein expression to be obtained.
III Protocol
1. Use BamHI and XhoI to insert eGFP fragment into the pET28 plasmid.
2. Linearize pET28-eGFP plasmid by PCR. (WT: F-CCCGCTGCAGTAATACGACTCACTATAGGGAGAGGAATTGTGAGCGGATAACAA / R- GCGGTGGACTGCAGCAACTCAGCTTCCTTTCGGGCT ; Mu: F-CCCGCTGCAGTAATACGACTCACWNNNGSRRNNGGAATTGTGAGCGGATAACAA / R- GCGGTGGACTGCAGCAACTCAGCTTCCTTTCGGGCT )
3. Cyclization the plasmid by PstI and ligase.
4. Transform the plasmids into E. coli BL21(DE3), add 20μl 100mM IPTG to the plate before coating the plate.
5. Incubate at 37℃ and observe colony color to screen for strong mutants preliminarily.
6. Pick greener single colonies, add the colonies into 3ml Lb, incubate at 37℃ in a shaker for 6-8h to dilute the IPTG introduced when picking single colonies.
7. Add 10μl germ solution from last step into 3ml Lb, incubate at 37℃ in a shaker until OD600 reaches 0.6.
8. Add IPTG to final concentrations 0.5mM, and induce for 4h at 18℃.
9. Measure the fluorescence (excitation wavelength: 485 nm; detection wavelength: 528 nm) and OD600.
IV Reference
[1] Ikeda R A, Ligman C M, Warshamana S, et al. T7 promoter contacts essential for promoter activity in vivo[J]. Nucleic Acids Research, 1992, 20(10): 2517-2524. [2] Paul S, Stang A, Lennartz K, Tenbusch M, Uberla K. Selection of a T7 promoter mutant with enhanced in vitro activity by a novel multi-copy bead display approach for in vitro evolution[J]. Nucleic Acids Research, 2013, 41(1):e29.