Difference between revisions of "Part:BBa K3239010:Experience"
BrianSebZhou (Talk | contribs) (→User Reviews) |
BrianSebZhou (Talk | contribs) (→User Reviews) |
||
| Line 40: | Line 40: | ||
*OD600 is used as an indicator of yeast concentration in the experiment. | *OD600 is used as an indicator of yeast concentration in the experiment. | ||
*Measured unit cell GFP production is the ratio of the GFP mean fluorescence intensities to the 0D600 absorbance measured by the plate reader. It shall serve as an indicator of the expression level of GFP at the sampled time point. | *Measured unit cell GFP production is the ratio of the GFP mean fluorescence intensities to the 0D600 absorbance measured by the plate reader. It shall serve as an indicator of the expression level of GFP at the sampled time point. | ||
| − | *Aggregate unit cell GFP production is the calculated total unit cell GFP fluorescence intensity. It accounts for the GFP that has degraded over the incubation period to more accurately reflect the production rate. | + | *Aggregate unit cell GFP production is the calculated total unit cell GFP fluorescence intensity. It accounts for the GFP that has degraded over the incubation period to more accurately reflect the aggregate production rate. |
*Total GFP production is the product of the yeast concentration and the aggregate unit cell GFP. It reflects the total GFP production of the reaction system of the strain. | *Total GFP production is the product of the yeast concentration and the aggregate unit cell GFP. It reflects the total GFP production of the reaction system of the strain. | ||
*Total GFP production per gram methanol is the ratio of the total GFP production to the total methanol consumption. It reflects the overall conversion rate of methanol to the product. | *Total GFP production per gram methanol is the ratio of the total GFP production to the total methanol consumption. It reflects the overall conversion rate of methanol to the product. | ||
Revision as of 09:28, 16 October 2019
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K3239010
This construct is designed to moderately up-regulate the AOX1 promoter (pAOX1) activity in P. pastoris GS115. It is to be homologously recombined into the pAOX1-GFP strain. Upon methanol induction, pPRM1 is activated, upregulating the expression of both the homogeneous Prm1 (which then self-upregulates itself) and the heterogeneous Mit1. This leads to a very strong activation of the AOX1 promoter.
Prm1 then activates pMIT1, upregulating the expression of the homogeneous Mit1. Unlike wildtype P. pastoris GS115 where Mit1 expression is then only upregulated by Prm1, in the constructed pAPM1-pAOX1-GFP strain, the heterogeneous Mit1 is also self-downregulated due to the downregulation of pPRM1 by Mit1. This corresponds to a much stronger activation pAOX1 compared to wildtype P. pastoris GS115 upon initial methanol induction. In later stages, pAOX1 activation might be weaker due to an accumulation of Mit1 that suppresses pPRM1. After Mit1 is fully degraded, however, pPRM1 inhibition shall be removed and pAOX1 shall be again strongly induced. Therefore the regulation of pAOX1 activity should be more like a cyclical process.
We included self-inhibition of Mit1 in the construct due to the fact that earlier research has demonstrated that if Mit1 is strongly self-upregulated by pAOX1 (which is activated by Mit1 and Prm1), the yeast cell will not survive. This suggests that Mit1 is slightly cytotoxic and its expression should be moderated at an appropriate level.
Given that in constructed strains, pAOX1 not only expresses the yEGFP3 reporter gene, but also the homogenous AOX1 (alcohol oxidase 1) gene, the pAPM1-pAOX1-GFP strain should not only have a higher GFP yield compared to the pAOX1-GFP strain (pAOX1-GFP homologously recombined into wildtype P. pastoris GS115) but also exhibit higher growth rates since it should be more capable of metabolizing methanol.
User Reviews
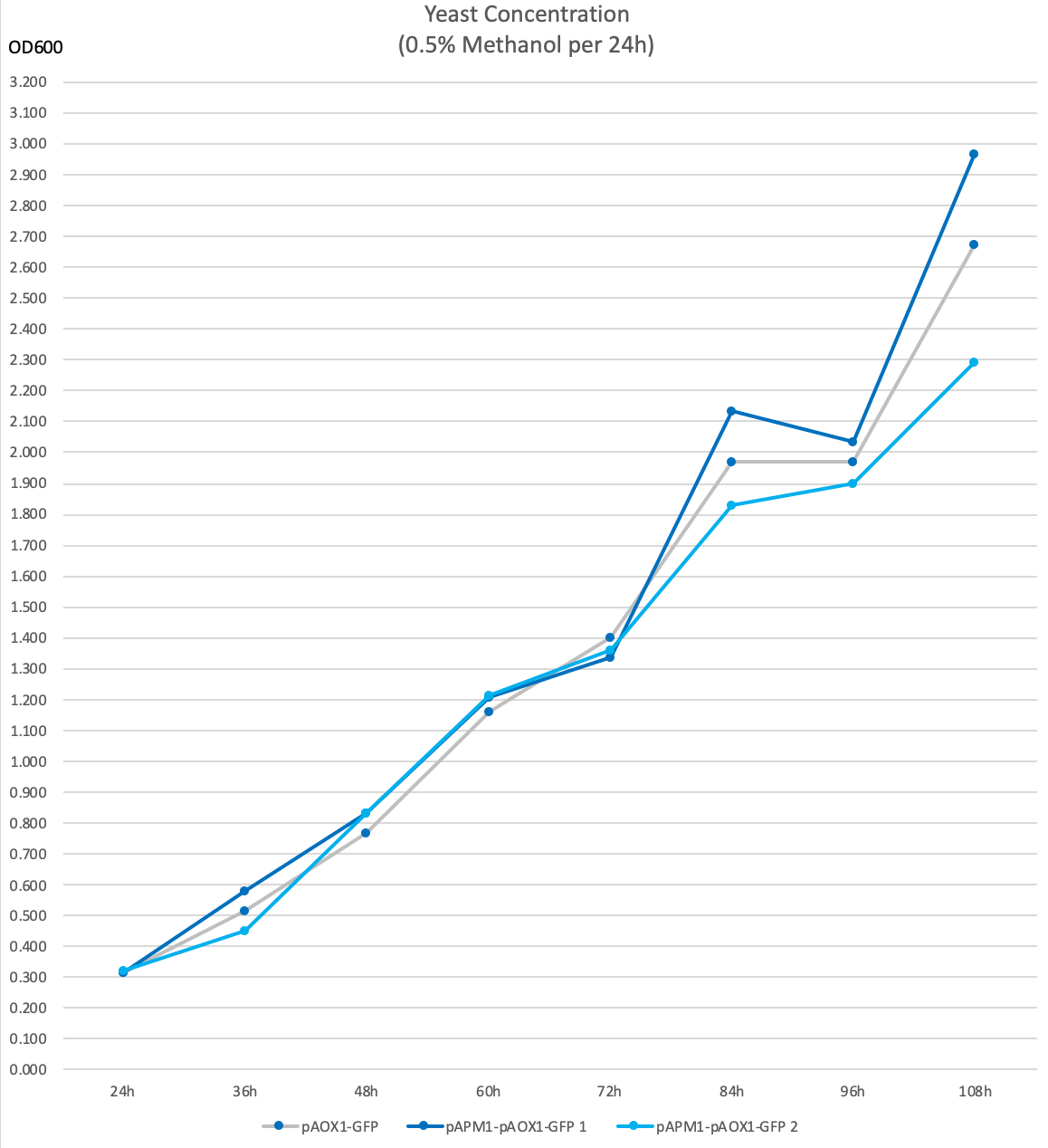
We labeled our strains containing this construct pAPM1-pAOX1-GFP and compared it to pAOX1-GFP, the positive control of our experiment.
Two identical pAPM1-pAOX1-GFP strains were used in our experiment, labeled 1 and 2 respectively.
Methods
- The following data were acquired after 108h 50mL YNM (1.34% yeat nitrogenous base with 0.5% methanol, 50µg/mL histidine and 40µg/mL biotin) incubation, sampled every 12h. The starting concentration was 1 OD600.
- 0.5%Methanol was added to the media every 24 hours to maintain the methanol concentration.
- A short-term methanol induction experiment was performed after the 108h incubation to characterize the instant response to methanol induction after the yeast cells are accustomed to methanol media, hence indicating the overall induction activity of the construct. The yeast cells were collected, rinsed and stored at 4˚C overnight to allow GFP to fully degrade. The strains were then incubated in higher-concentration 50mL YNM media (1.34% yeat nitrogenous base with 0.75% methanol, 50µg/mL histidine and 40µg/mL biotin) for 12 hours and sampled every 2 hours. The starting concentration was 3 OD600.
- Three parallels were performed for each strain.
- We used a Biotek Synergy 2 plate reader to measure the GFP mean fluorescence intensities and the OD600 absorbance of our samples.
- Methanol concentration measurements were performed with a Shenzhen Sieman M100 Biosensors Analyzer.
Data
- OD600 is used as an indicator of yeast concentration in the experiment.
- Measured unit cell GFP production is the ratio of the GFP mean fluorescence intensities to the 0D600 absorbance measured by the plate reader. It shall serve as an indicator of the expression level of GFP at the sampled time point.
- Aggregate unit cell GFP production is the calculated total unit cell GFP fluorescence intensity. It accounts for the GFP that has degraded over the incubation period to more accurately reflect the aggregate production rate.
- Total GFP production is the product of the yeast concentration and the aggregate unit cell GFP. It reflects the total GFP production of the reaction system of the strain.
- Total GFP production per gram methanol is the ratio of the total GFP production to the total methanol consumption. It reflects the overall conversion rate of methanol to the product.
- For teams wishing to compare their data to ours, we've provided our raw data along with our plate reader calibration data in our wiki. Feel free to give it a check!
Conclusions and Discussions
The main indicators of the GFP production efficiency in our experiment shall be the total GFP production per gram methanol during the 108h incubation period and the measured unit cell GFP production during the short-term methanol induction period, as the former demonstrates the overall conversion rate from the substrate to the desired product over a fairly extended period, while the latter demonstrates the instantaneous expression efficiency of the construct after stabilization in the methanol media.
For the pAPM1-pAOX1-GFP strain, To yield more representative data, however, incubation over a further extended period in a more stable system (e.g. one-week-long chemostat incubation) might be necessary.
UNIQa1a3893a9be00ced-partinfo-00000000-QINU UNIQa1a3893a9be00ced-partinfo-00000001-QINU