Difference between revisions of "Part:BBa K1334002"
| Line 27: | Line 27: | ||
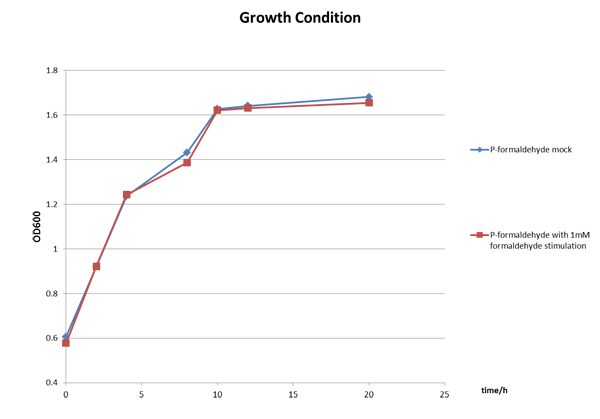
Figure 3 The growth condition of E.coli strain DH5a after formaldehyde stimulation. | Figure 3 The growth condition of E.coli strain DH5a after formaldehyde stimulation. | ||
| − | + | [[File:Figure 5 The growth condition of E.coli strain DH5a after formaldehyde stimulation.png|center]] | |
We detected the sensitivity and tolerance of the promoter to formaldehyde using BL21 as chassis. We cultured E.coli BL21 transformed with the plasmid (P-formaldehyde plus GFP)and added different concentration formaldehyde to different groups when the OD600 = 1. Then we collected different group bacteria at certain time to measure the fluorescence and the absorbance. From the Fig. We found that 0.5mM formaldehyde is the best concentration for expression reporter protein GFP. | We detected the sensitivity and tolerance of the promoter to formaldehyde using BL21 as chassis. We cultured E.coli BL21 transformed with the plasmid (P-formaldehyde plus GFP)and added different concentration formaldehyde to different groups when the OD600 = 1. Then we collected different group bacteria at certain time to measure the fluorescence and the absorbance. From the Fig. We found that 0.5mM formaldehyde is the best concentration for expression reporter protein GFP. | ||
Revision as of 08:39, 15 October 2019
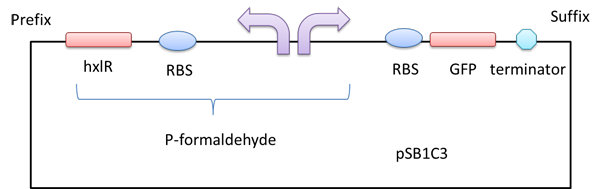
HxlR+P-formaldehyde
A right promoter which can sense formaldehyde.There is a left promoter which can erpress the HxlR protein.The HxlR protein can enhance the function of the P-formaldehyde.
Usage and Biology
A gfp reporter gene was added downstream so that we can know whether the P-formaldehyde works or not in the presence of formaldehyde by detecting the relative fluorescence intensity.
Figure 1 A GFP reporter is added downstream of the formaldehyde promoter.
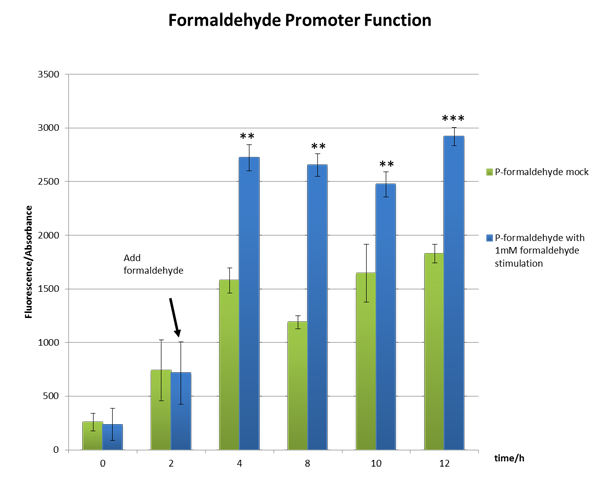
We incubated E.coli strain DH5a with the above plasmid (P-formaldehyde plus GFP)and stimulated the experimental group with 1mM formaldehyde when the OD600 value reached approximately one, which means E.coli comes into mid-log phase. And then we collected bacteria at certain time to measure the fluorescence and the absorbance.
From the Figure 2, we can see significant increase in relative fluorescence intensity after formaldehyde stimulation, which shows that our coloration system works well.
The Growth Condition curve in Figure 3 shows that the difference of the fluorescence/absorbance is not generated by the absorbance difference.
Figure 2 The function test of the formaldehyde promoter which shows our part BBa_K1334002 works well。
Figure 3 The growth condition of E.coli strain DH5a after formaldehyde stimulation.
We detected the sensitivity and tolerance of the promoter to formaldehyde using BL21 as chassis. We cultured E.coli BL21 transformed with the plasmid (P-formaldehyde plus GFP)and added different concentration formaldehyde to different groups when the OD600 = 1. Then we collected different group bacteria at certain time to measure the fluorescence and the absorbance. From the Fig. We found that 0.5mM formaldehyde is the best concentration for expression reporter protein GFP.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]