Difference between revisions of "Part:BBa K3147006"
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K3147006 short</partinfo> | <partinfo>BBa_K3147006 short</partinfo> | ||
| + | |||
| + | ===I : parts BBa_K3147006 (MBP-TEVcs-TEVPE10) function :=== | ||
The Montpellier 2019 team made this construction in order to be able to compare the basal activity of the TEV protease (BBa_ K1319004) with the mutant TEV protease called TEVPE10, we were able to find the mutant TEV sequences in this article [3]. | The Montpellier 2019 team made this construction in order to be able to compare the basal activity of the TEV protease (BBa_ K1319004) with the mutant TEV protease called TEVPE10, we were able to find the mutant TEV sequences in this article [3]. | ||
This construction was used as a control within the Karma project presented by the iGEM Montpellier 2019 team. It produces a mutant TEV fused to an MBP with, in between a modified TEV cutting site to increase the mutant's activity. MBP increases the solubility of the fusion protein [1] preventing the aggregation of the protein of interest ; the sequence of the produced MBP does not have a signal peptide which keeps the protein in the cytosol. The TEV cutting site allows to separate MBP from TEV once the protein is produced, indeed the TEV protease is capable of self-cleavage [2] to remove MBP. | This construction was used as a control within the Karma project presented by the iGEM Montpellier 2019 team. It produces a mutant TEV fused to an MBP with, in between a modified TEV cutting site to increase the mutant's activity. MBP increases the solubility of the fusion protein [1] preventing the aggregation of the protein of interest ; the sequence of the produced MBP does not have a signal peptide which keeps the protein in the cytosol. The TEV cutting site allows to separate MBP from TEV once the protein is produced, indeed the TEV protease is capable of self-cleavage [2] to remove MBP. | ||
| + | |||
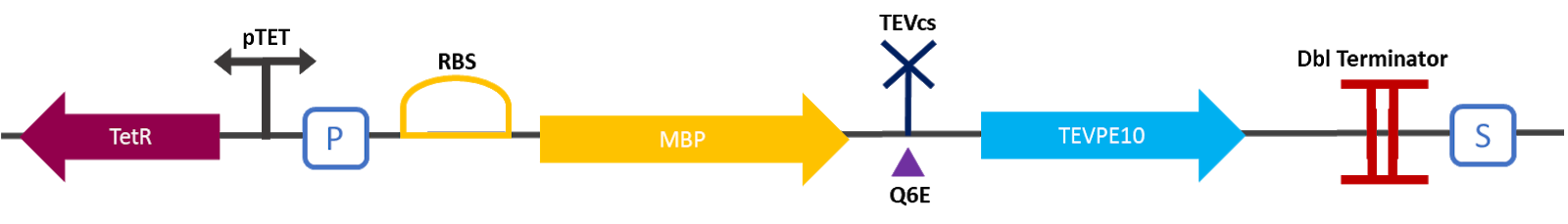
| + | [[File:designK3147006.png|650px]] | ||
| + | |||
| + | Figure 1: Construct Design: MBP-TEVcs-TEVPE10 with modified TEV cutting site. The objective of this construction is to be able to compare the activity of a TEV protease with this mutant TEV protease. | ||
| + | |||
| + | ===II. Proof of function=== | ||
| + | |||
| + | The construction was cloned by Gibson Assembly in a pOUT18 backbone under the control of a TET ON promoter, in order to control its expression. The TEV cutting site used is the mutated site: ENLYFE/G this mutation allows theoretically the mutant TEV to better cleave this sequence according to this source [3]. | ||
| + | |||
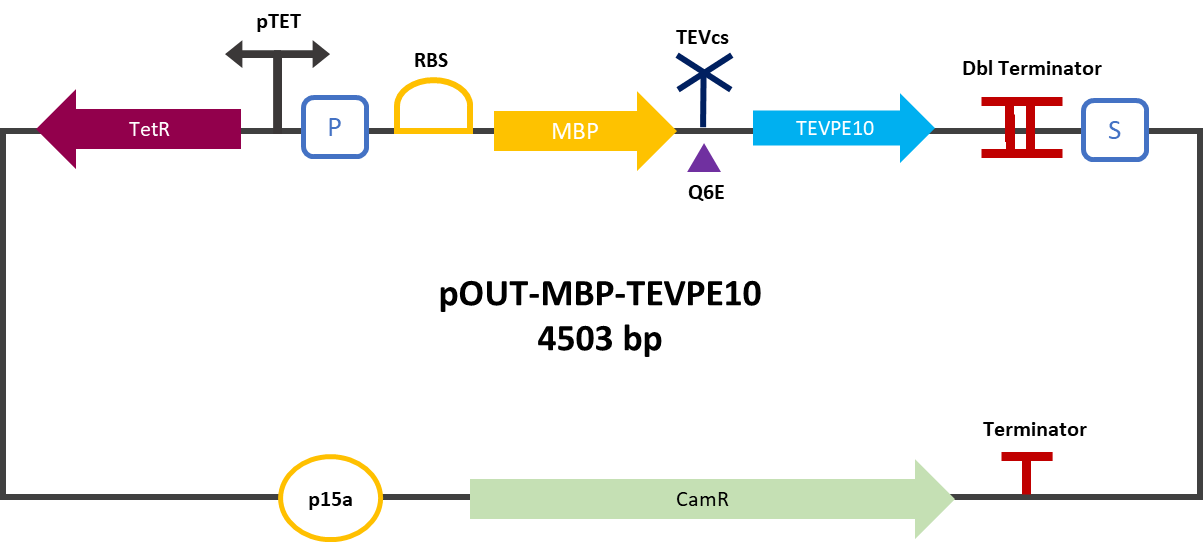
| + | [[File:plasmideK3147006.png|450px]] | ||
| + | |||
| + | Figure 2 : MBP-TEVPE10 reporter gene in its backbone pOUT18. | ||
| + | |||
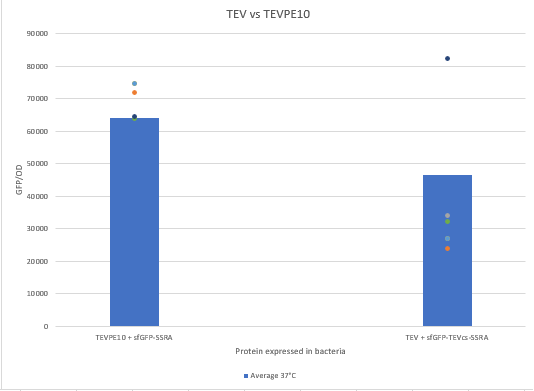
| + | The experimental approach that has been used to test the protease activity is to compare the fluorescence restoration rate of sfGFP compared to MBP-TEV and MBP-TEVPE10. In this experiment, basal controls of maximum and minimum fluorescence of the reporter genes were used. Fluorescence data are obtained from Plate Reader at 37°C. The protease is expressed by inducing the TET promoter with 50ng/mL of aTc (anhydrotetracycline). | ||
| + | |||
| + | [[File:resultK3147006.png|500px]] | ||
| + | |||
| + | Figure 2 : Comparison of the activity of TEVPE10 to the TEV WT by measuring the fluorescence of a reporter that needs to be cutted from a proteolysis tag | ||
| + | |||
| + | We can see that the TEVPE10 is more efficient than the TEV WT. We think it’s because our TEV WT isn’t the one in the article where we found this mutant. | ||
| + | |||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Revision as of 06:54, 14 October 2019
mutant TEV (PE10) protease with TEV-cleavable maltose binding protein
I : parts BBa_K3147006 (MBP-TEVcs-TEVPE10) function :
The Montpellier 2019 team made this construction in order to be able to compare the basal activity of the TEV protease (BBa_ K1319004) with the mutant TEV protease called TEVPE10, we were able to find the mutant TEV sequences in this article [3]. This construction was used as a control within the Karma project presented by the iGEM Montpellier 2019 team. It produces a mutant TEV fused to an MBP with, in between a modified TEV cutting site to increase the mutant's activity. MBP increases the solubility of the fusion protein [1] preventing the aggregation of the protein of interest ; the sequence of the produced MBP does not have a signal peptide which keeps the protein in the cytosol. The TEV cutting site allows to separate MBP from TEV once the protein is produced, indeed the TEV protease is capable of self-cleavage [2] to remove MBP.
Figure 1: Construct Design: MBP-TEVcs-TEVPE10 with modified TEV cutting site. The objective of this construction is to be able to compare the activity of a TEV protease with this mutant TEV protease.
II. Proof of function
The construction was cloned by Gibson Assembly in a pOUT18 backbone under the control of a TET ON promoter, in order to control its expression. The TEV cutting site used is the mutated site: ENLYFE/G this mutation allows theoretically the mutant TEV to better cleave this sequence according to this source [3].
Figure 2 : MBP-TEVPE10 reporter gene in its backbone pOUT18.
The experimental approach that has been used to test the protease activity is to compare the fluorescence restoration rate of sfGFP compared to MBP-TEV and MBP-TEVPE10. In this experiment, basal controls of maximum and minimum fluorescence of the reporter genes were used. Fluorescence data are obtained from Plate Reader at 37°C. The protease is expressed by inducing the TET promoter with 50ng/mL of aTc (anhydrotetracycline).
Figure 2 : Comparison of the activity of TEVPE10 to the TEV WT by measuring the fluorescence of a reporter that needs to be cutted from a proteolysis tag
We can see that the TEVPE10 is more efficient than the TEV WT. We think it’s because our TEV WT isn’t the one in the article where we found this mutant.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 77
Illegal SapI.rc site found at 1868