Difference between revisions of "Part:BBa K3147005"
| Line 3: | Line 3: | ||
<partinfo>BBa_K3147005 short</partinfo> | <partinfo>BBa_K3147005 short</partinfo> | ||
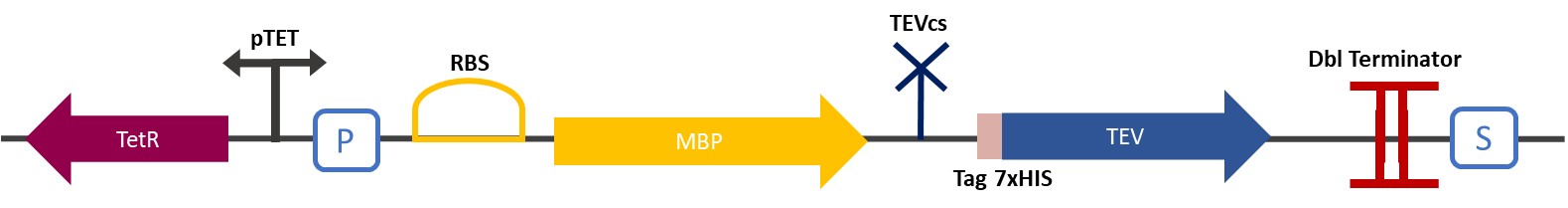
| − | The Montpellier 2019 team made this construction in order to be able to compare the basal activity of the TEV protease (BBa_ K1319004) against the same protease fused to a nanobody. This construction was used as a control within the framework of the KARMA project. This construction produces a TEV merged with an MBP separated by a TEV cutting site (BBa_ J18918). MBP increases the solubility [1] of the fusion protein preventing the aggregation of the protein of interest, this stabilizes expression, the sequence of the produced MBP does not have a signal peptide which allows the protein to be kept in the cytosol. The TEV cutting site allows to separate MBP from the TEV once the protein is produced, indeed the TEV protease is capable of self-cleavage to remove the MBP [2]. An affinity tag 7 histidine is added at the N-ter of the TEV protease and can enable the separation activity of the TEV to be detected by a Western-Blot. | + | ===I : parts BBa_K3147005 (MBP-TEVcs-TEV) function :=== |
| + | |||
| + | The Montpellier 2019 team made this construction in order to be able to compare the basal activity of the TEV protease (BBa_ K1319004) against the same protease fused to a nanobody. This construction was used as a control within the framework of the KARMA project. This construction produces a TEV (BBa_K1319004) merged with an MBP separated by a TEV cutting site (BBa_ J18918). MBP increases the solubility [1] of the fusion protein preventing the aggregation of the protein of interest, this stabilizes expression, the sequence of the produced MBP does not have a signal peptide which allows the protein to be kept in the cytosol. The TEV cutting site allows to separate MBP from the TEV once the protein is produced, indeed the TEV protease is capable of self-cleavage to remove the MBP [2]. An affinity tag 7 histidine is added at the N-ter of the TEV protease and can enable the separation activity of the TEV to be detected by a Western-Blot. | ||
| + | |||
| + | |||
| + | [[File:designK3147005.png|650ppx]] | ||
| + | |||
| + | |||
| + | Figure 1 : Construct Design: MBP-TEVcs-TEV with TEV cutting site. The objective of the construction is to be able to compare the activity of a TEV protease alone against a TEV protease fused to a VHH. | ||
| + | |||
| + | ===II. Proof of function=== | ||
| + | |||
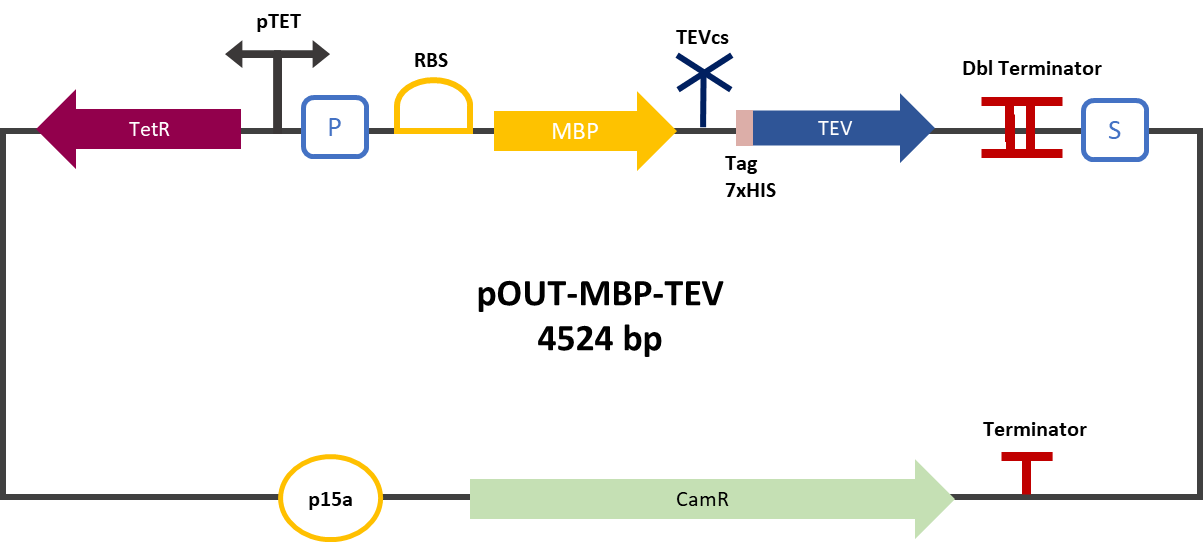
| + | The experimental approach to test protease activity is to compare the fluorescence restoration rate of sfGFP comparing MBP-TEV and MBP-TEV fused to a VHH. The construction was cloned by Gibson Assembly in a backbone pOUT18 under the control of a TET ON promoter, in order to control its expression. The TEV cleavage site used is the classic ENLYFQ site, MBP stabilizes the expression and increases the solubility of the fusion protein. The TEV protease used is optimized for expression in E. coli. The sequence contains an cleavage mutation "S219V anti-self-cleavage". | ||
| + | |||
| + | |||
| + | [[File:plasmideK3147005.png|450ppx]] | ||
| + | |||
| + | Figure 2: MBP-TEV reporter gene in its backbone pOUT18 | ||
| + | |||
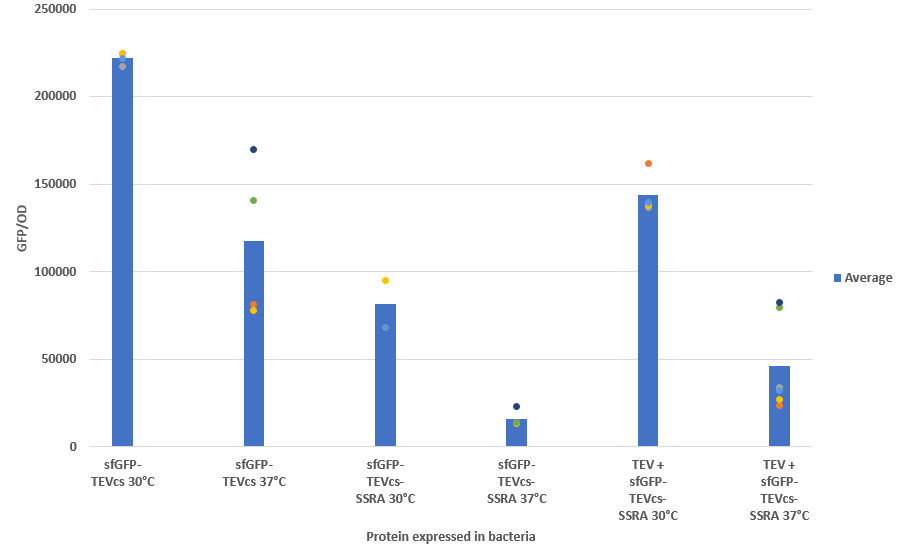
| + | In order to carry out this experiment we used e.coli bacteria of the NEB10B strain. Fluorescence data are obtained by a Plate Reader at 30°C and 37°C. The protease is expressed by inducing the TET promoter with 50ng/mL of aTc (anhydrotetracycline). In this experiment, basal controls of maximum and minimum fluorescence of reporter genes were used. | ||
| + | |||
| + | |||
| + | [[File:result2K3147005.png|450ppx]] | ||
| + | |||
| + | Figure 3 : Efficiency of TEV by measuring the fluorescence recovery with a reporter linked to a proteolysis tag | ||
| + | |||
| + | ==Reference== | ||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Revision as of 21:15, 13 October 2019
TEV protease with TEV-cleavable maltose binding protein
I : parts BBa_K3147005 (MBP-TEVcs-TEV) function :
The Montpellier 2019 team made this construction in order to be able to compare the basal activity of the TEV protease (BBa_ K1319004) against the same protease fused to a nanobody. This construction was used as a control within the framework of the KARMA project. This construction produces a TEV (BBa_K1319004) merged with an MBP separated by a TEV cutting site (BBa_ J18918). MBP increases the solubility [1] of the fusion protein preventing the aggregation of the protein of interest, this stabilizes expression, the sequence of the produced MBP does not have a signal peptide which allows the protein to be kept in the cytosol. The TEV cutting site allows to separate MBP from the TEV once the protein is produced, indeed the TEV protease is capable of self-cleavage to remove the MBP [2]. An affinity tag 7 histidine is added at the N-ter of the TEV protease and can enable the separation activity of the TEV to be detected by a Western-Blot.
Figure 1 : Construct Design: MBP-TEVcs-TEV with TEV cutting site. The objective of the construction is to be able to compare the activity of a TEV protease alone against a TEV protease fused to a VHH.
II. Proof of function
The experimental approach to test protease activity is to compare the fluorescence restoration rate of sfGFP comparing MBP-TEV and MBP-TEV fused to a VHH. The construction was cloned by Gibson Assembly in a backbone pOUT18 under the control of a TET ON promoter, in order to control its expression. The TEV cleavage site used is the classic ENLYFQ site, MBP stabilizes the expression and increases the solubility of the fusion protein. The TEV protease used is optimized for expression in E. coli. The sequence contains an cleavage mutation "S219V anti-self-cleavage".
Figure 2: MBP-TEV reporter gene in its backbone pOUT18
In order to carry out this experiment we used e.coli bacteria of the NEB10B strain. Fluorescence data are obtained by a Plate Reader at 30°C and 37°C. The protease is expressed by inducing the TET promoter with 50ng/mL of aTc (anhydrotetracycline). In this experiment, basal controls of maximum and minimum fluorescence of reporter genes were used.
Figure 3 : Efficiency of TEV by measuring the fluorescence recovery with a reporter linked to a proteolysis tag
Reference
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 379
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]