Difference between revisions of "Part:BBa K1033933:Experience"
(→Applications of BBa_K1033933) |
(→Applications of BBa_K1033933) |
||
| Line 9: | Line 9: | ||
2019 iGEM team Linkoping Sweden validated this part.<br><br> | 2019 iGEM team Linkoping Sweden validated this part.<br><br> | ||
<b>Growth of p.cons-asPink</b><br> | <b>Growth of p.cons-asPink</b><br> | ||
| − | The agar plate contains | + | The agar plate contains E.coli (BL21(DE3)) with a p.Cons-AsPink plasmid (<partinfo>BBa_K3182100</partinfo>). The promotor is constitutive which will express AsPink and makes the colonies pink. This can be used to determine whether a ligation was successful. |
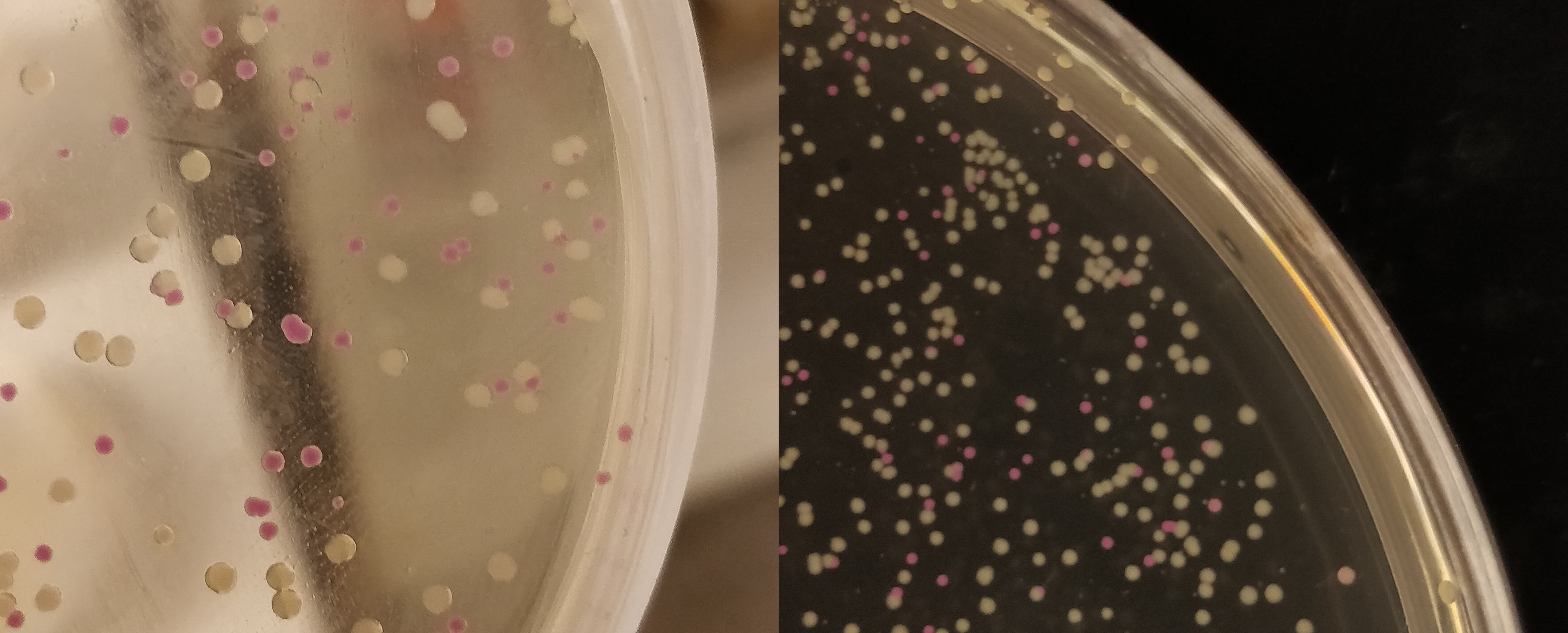
[[File:T--Linkoping_Sweden--pinkwhite156.jpeg|700px|thumb|left|<b>Figure 2.</b> E. coli (BL21) cells used for pink-white screening, the cells were incubated for 16 hours in 37 degrees Celsius. <partinfo>BBa_K3182100</partinfo> was cut with BamHI and PstI to remove pCons-AsPink and <partinfo>BBa_K3182006</partinfo> (magainin 2) and <partinfo>BBa_K3182104</partinfo> (CHAP) was ligated into the plasmid. The white colonies indicate a successful ligation. All the colonies that were later colony screened with PCR amplification of the insert and the ampified strand was run on an agarose gel. The gel implied that all screened colonies was successful, i.e. contained <partinfo>BBa_K3182100</partinfo> with magainin 2 / CHAP instead of pCons-AsPink. ]] | [[File:T--Linkoping_Sweden--pinkwhite156.jpeg|700px|thumb|left|<b>Figure 2.</b> E. coli (BL21) cells used for pink-white screening, the cells were incubated for 16 hours in 37 degrees Celsius. <partinfo>BBa_K3182100</partinfo> was cut with BamHI and PstI to remove pCons-AsPink and <partinfo>BBa_K3182006</partinfo> (magainin 2) and <partinfo>BBa_K3182104</partinfo> (CHAP) was ligated into the plasmid. The white colonies indicate a successful ligation. All the colonies that were later colony screened with PCR amplification of the insert and the ampified strand was run on an agarose gel. The gel implied that all screened colonies was successful, i.e. contained <partinfo>BBa_K3182100</partinfo> with magainin 2 / CHAP instead of pCons-AsPink. ]] | ||
| Line 18: | Line 18: | ||
<b>CBD-asPink bindning capacity</b><br> | <b>CBD-asPink bindning capacity</b><br> | ||
| − | To test the bindning capacity of CBD-asPink (<partinfo>BBa_K3182000</partinfo>) the | + | To test the bindning capacity of CBD-asPink (<partinfo>BBa_K3182000</partinfo>) the microbial cellulose bandage was suspended into sonicated lysate of BL21(DE3) with the expressed fusion protein (see <b><I>Figure 1</I></b>) and incubated for 30 minutes. |
| − | The bandage was then washed thrice in 70% ethanol which confirmed that the bindning of CDB-asPink was | + | The bandage was then washed thrice in 70% ethanol which confirmed that the bindning of CDB-asPink was specific for the cellulose bandage.] |
<br><br> | <br><br> | ||
| Line 27: | Line 27: | ||
<b> CBD-asPink with thrombin cleavage</b><br> | <b> CBD-asPink with thrombin cleavage</b><br> | ||
| − | After the washes, human thrombin and cleavage buffer was | + | After the washes, human thrombin and cleavage buffer was added to the bandage with bound CBD-asPink to test the release mechanism of the fusion protein. The bandage was then incubated with the solution for 16 hours on an end to end rotator together with an negative control containing cellulose bandage and only cleavage buffer. |
| − | After the incubation, the supernatant containing thrombin | + | After the incubation, the supernatant containing thrombin was pink while the negative control containing only cleavage buffer was transparent with a clear pink cellulose bandage (see <b><I>Figure 2</I></b>). This indicates that the release mechanism work and the AsPink protein has successfully been released from the bandage into the supernatant. |
<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
<br> | <br> | ||
Revision as of 13:21, 4 September 2019
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K1033933
2019 iGEM team Linkoping Sweden
2019 iGEM team Linkoping Sweden validated this part.
Growth of p.cons-asPink
The agar plate contains E.coli (BL21(DE3)) with a p.Cons-AsPink plasmid (BBa_K3182100). The promotor is constitutive which will express AsPink and makes the colonies pink. This can be used to determine whether a ligation was successful.
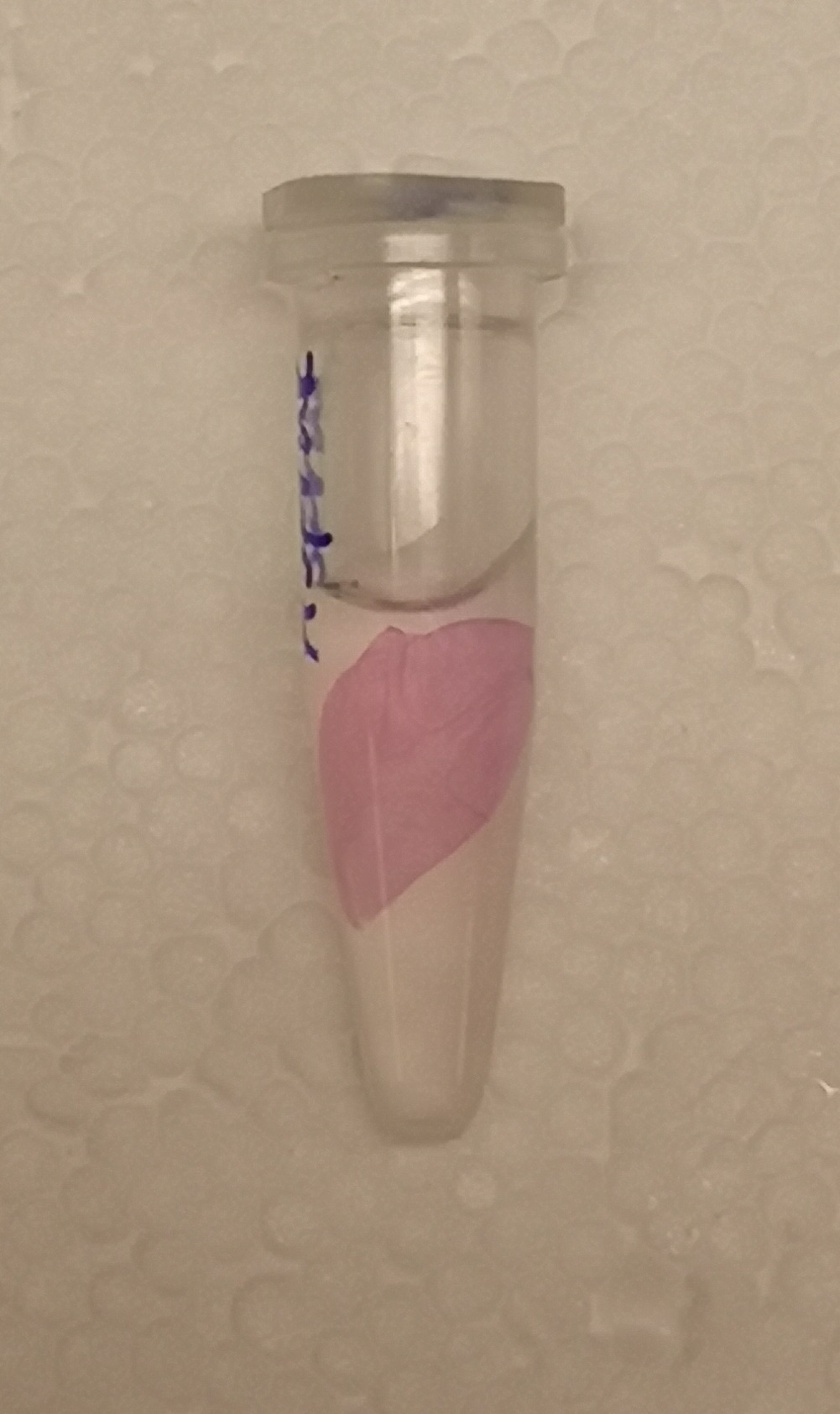
CBD-asPink bindning capacity
To test the bindning capacity of CBD-asPink (BBa_K3182000) the microbial cellulose bandage was suspended into sonicated lysate of BL21(DE3) with the expressed fusion protein (see Figure 1) and incubated for 30 minutes.
The bandage was then washed thrice in 70% ethanol which confirmed that the bindning of CDB-asPink was specific for the cellulose bandage.]
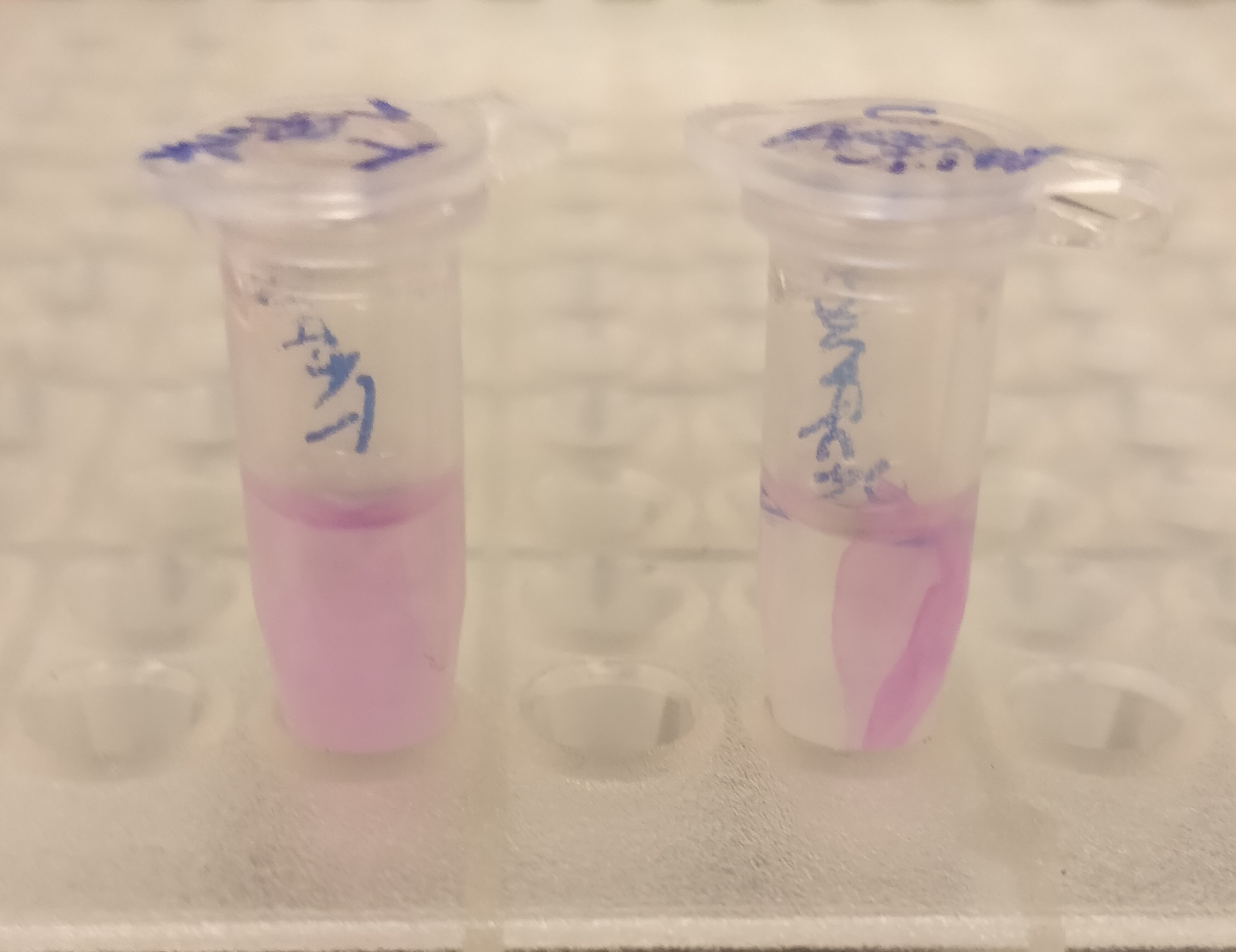
CBD-asPink with thrombin cleavage
After the washes, human thrombin and cleavage buffer was added to the bandage with bound CBD-asPink to test the release mechanism of the fusion protein. The bandage was then incubated with the solution for 16 hours on an end to end rotator together with an negative control containing cellulose bandage and only cleavage buffer.
After the incubation, the supernatant containing thrombin was pink while the negative control containing only cleavage buffer was transparent with a clear pink cellulose bandage (see Figure 2). This indicates that the release mechanism work and the AsPink protein has successfully been released from the bandage into the supernatant.
User Reviews
UNIQ5420103686219384-partinfo-00000006-QINU UNIQ5420103686219384-partinfo-00000007-QINU