Difference between revisions of "Part:BBa K2627000"
| Line 6: | Line 6: | ||
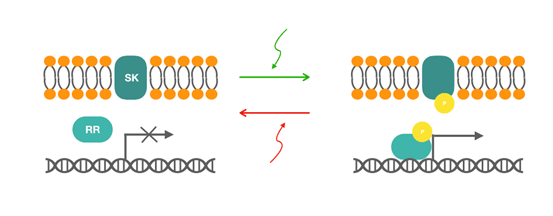
CcaS protein belongs to green/red light sensing two-component system. The two-component system consists of the membrane-associated histidine kinase CcaS and its response regulator CcaR. The activating information stored in light is captured by phytochromes in situ. In phytochromes, a bilin-chromophore (in this case phycocyanobilin) binds at a conserved cysteine within an N-terminal GAF (cyclic GMP phosphodiesterase, adenylyl cyclase, FhlA) domain and imparts reversible photoactivation of signaling activity with maximal responses to 535-nm (green) and 672-nm (red) light. Absorption of green light increases the rate of CcaS autophosphorylation, phosphorylation of CcaR by phosphate transferring, and transcription from the promoter of the phycobilisome linker protein cpcG2, while absorption of red light reverses this process. The lower leakiness is reported to be acquired after removing the second putative promoter in cpcG2, which is thought to be constitutive and contributes to leakiness and low dynamic range. | CcaS protein belongs to green/red light sensing two-component system. The two-component system consists of the membrane-associated histidine kinase CcaS and its response regulator CcaR. The activating information stored in light is captured by phytochromes in situ. In phytochromes, a bilin-chromophore (in this case phycocyanobilin) binds at a conserved cysteine within an N-terminal GAF (cyclic GMP phosphodiesterase, adenylyl cyclase, FhlA) domain and imparts reversible photoactivation of signaling activity with maximal responses to 535-nm (green) and 672-nm (red) light. Absorption of green light increases the rate of CcaS autophosphorylation, phosphorylation of CcaR by phosphate transferring, and transcription from the promoter of the phycobilisome linker protein cpcG2, while absorption of red light reverses this process. The lower leakiness is reported to be acquired after removing the second putative promoter in cpcG2, which is thought to be constitutive and contributes to leakiness and low dynamic range. | ||
| − | |||
[[File:T--SDU-China--3410.png|800px|center]] | [[File:T--SDU-China--3410.png|800px|center]] | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Revision as of 17:12, 17 October 2018
Advanced CcaS with PAS domain knocked out
Usage and Biology
CcaS protein belongs to green/red light sensing two-component system. The two-component system consists of the membrane-associated histidine kinase CcaS and its response regulator CcaR. The activating information stored in light is captured by phytochromes in situ. In phytochromes, a bilin-chromophore (in this case phycocyanobilin) binds at a conserved cysteine within an N-terminal GAF (cyclic GMP phosphodiesterase, adenylyl cyclase, FhlA) domain and imparts reversible photoactivation of signaling activity with maximal responses to 535-nm (green) and 672-nm (red) light. Absorption of green light increases the rate of CcaS autophosphorylation, phosphorylation of CcaR by phosphate transferring, and transcription from the promoter of the phycobilisome linker protein cpcG2, while absorption of red light reverses this process. The lower leakiness is reported to be acquired after removing the second putative promoter in cpcG2, which is thought to be constitutive and contributes to leakiness and low dynamic range.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 606
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 1397
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Results
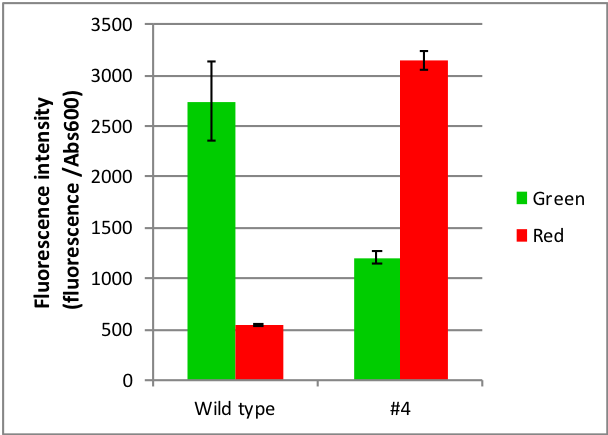
Figure 1: Characterization of part BBa_K2627000 and BBa_K592001. Part BBa_K2627000 shows a different effect that the index under red light is higher than the index under green light. The Fluorescence intensity is about 1207.4 under green light while it is about 3146.3 under red light. For this reason, we can treat the index under red light as expression index and treat the index under green light as leak index.