Difference between revisions of "Part:BBa K2812000"
(→References) |
|||
| Line 34: | Line 34: | ||
====References==== | ====References==== | ||
| − | 1) Guo, S., Stevens, C., Vance, T., Olijve, L., Graham, L., Campbell, R., . . . Davies, P. (2017). Structure of a 1.5-MDa adhesin that binds its Antarctic bacterium to diatoms and ice. Science Advances, 1-10. | + | 1) Guo, S., Stevens, C., Vance, T., Olijve, L., Graham, L., Campbell, R., . . . Davies, P. (2017). Structure of a 1.5-MDa adhesin that binds its Antarctic bacterium to diatoms and ice. ''Science Advances'', 1-10. |
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
Revision as of 01:23, 17 October 2018
Carbohydrate-binding domain from MpIBP
Carbohydrate-binding domain from the Marinomonas primoryensis ice-binding protein (MpIBP). It requires millimolar concentrations of calcium ions to properly fold into a globular β-fold. A coordinated calcium ion is used to bind sugar moieties, such as glucose.1 Unfolding of the domain can be induced by the addition of EDTA, which chelates the calcium ions and prevents binding of sugar moieties. It does not require the presence of other domains to fold and therefore it can be used as a modular protein domain to bind sugar moieties. TU Eindhoven 2018 used the carbohydrate-binding domain from MpIBP to tether E. coli bacteria to the glucose-based polymer dextran. Please visit our [http://2018.igem.org/Team:TU-Eindhoven wiki] for more information.
Usage and Biology
The Marinomonas primoryensis ice-binding protein (MpIBP) is a 1.5-MDa adhesin used by the Antarctic bacterium M. primoryensis to bind to ice and diatoms to position itself on top of the water column to access nutrients and oxygen. The carbohydrate-binding domain from this protein is used to bind extracellular polysaccharides to form microcolonies and to tether M. primoryensis to other photosynthetic microorganisms.
Characterisation by TU Eindhoven (2018)
Cloning
The biobrick has been characterized by the iGEM 2018 Eindhoven team. The carbohydrate-binding domain was synthesized by IDT. Both the linearized plasmid backbone pSB1C3 and the carbohydrate-binding domain were double digested by EcoRI and PstI and subsequently assembled via ligation after purification of the backbone. Successful transformation of the ligated product into NovaBlue E. coli cells was followed by a colony PCR using the VF2 and VR primers, to confirm the that the desired insert has been inserted into pSB1C3. The results of the colony PCR can be seen in figure 1. A length of 890 basepairs is expected for this construct, which corresponds with the bright band in lane 10 that is marked with a yellow box in figure 1. Due to synthesis problems at IDT, the construct delivered contained multiple DNA fragments that could not be separated, explaining the large amount of colonies with a wrong insert. The colony corresponding to lane 10 was cultured in LB medium and plasmid DNA was obtained via miniprep. This plasmid DNA was used for Sanger sequencing. It could be confirmed that the correct insert has been incorporated in pSB1C3.
Proof of Functionality
Experimental Setup
This experiment was performed to verify the calcium-dependent dextran binding capabilities of the MpIBP carbohydrate-binding domain. The experimental setup can be seen in figure 2:
The MpIBP carbohydrate-binding domain was expressed, isolated and purified from E. coli BL21 (DE3). Equimolar amounts of purified MpIBP carbohydrate-binding domain were incubated with dextran-based Sephadex beads (commonly used for size-exclusion chromatography) in the presence of 5mM CaCl2 (sample A) or 10mM EDTA (Sample B). Dextran is a glucose polymer, to which our MpIBP carbohydrate-binding domain should bind in the presence of millimolar concentrations of calcium. After incubation, each sample was centrifuged shortly at 8,000 rpm to pull the dextran-based Sephadex beads (and any dextran-bound proteins) down from the mixture and the supernatant was isolated. Next, the Sephadex beads were resuspended in buffer with CaCl2 (sample A) or with EDTA (sample B) and allowed to incubate. After incubation, the samples were centrifuged shortly at 8,000 rpm and the supernatant was isolated.
SDS samples were prepared from the dextran-based Sephadex beads after the final centrifugation (lane 1 & 4), supernatant from the first incubation (lane 2 & 5) and supernatant from the resuspension (lane 3 & 6). The samples were boiled at 95 degrees Celcius for 15 minutes. Subsequently, the samples were loaded on a SDS-PAGE gel and the gel was run at 120V for one hour. Anything bound to the dextran-based Sephadex beads will be released into the solution during the boiling with SDS.
Results
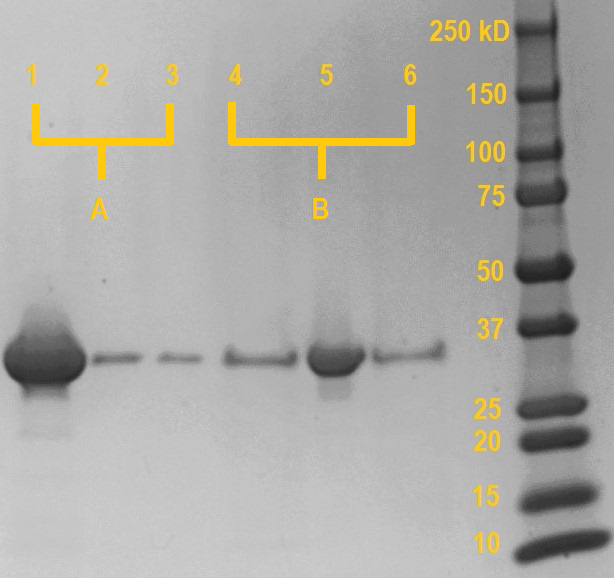
As can be seen on the SDS gel in figure 3, if the MpIBP carbohydrate-binding domain is incubated with dextran in the presence of millimolar concentrations of calcium, only a very small amount of protein remains unbound in the supernatant (lane 2; probably due to saturation of the dextran-based Sephadex beads). However, if the protein domain is incubated in the presence of EDTA, which chelates the calcium, almost all protein remains in unbound in the supernatant (lane 5).
In the subsequent washing step (lane 3 & 6), sample A is washed with buffer containing calcium and sample B is washed with buffer containing EDTA to wash away any protein with no affinity for dextran. For both sample A and B, the amount of non-specifically bound protein released during the washing is very minor and comparable. This shows that most proteins stay bound in the case of sample A, while in sample B most protein has already been removed during the first washing step.
Boiling the dextran-based Sephadex beads releases any protein still bound to the dextran-based Sephadex beads after washing. In the case of sample A (incubated in the presence of calcium), a very large amount of protein has remained bound to the dextran-based Sephadex beads during the washing and is released during boiling in SDS (lane 1). Conversely, the dextran-based Sephadex beads incubated with the MpIBP carboydrate-binding protein in the presence of EDTA release negligible amounts of protein compared to sample A after boiling in SDS, as almost all protein has not bound during the incubation due to the chelation of CaCl2.
Conclusion
The observations above demonstrate that the MpIBP carbohydrate-binding domain binds to dextran. The binding of the MpIBP carbohydrate-binding domain is calcium dependent and negligible binding occurs in the absence of calcium. EDTA can be used to chelate the calcium, inducing unfolding of the MpIBP and preventing binding to dextran. The isolated carbohydrate-binding domain of the 1.5 MDa MpIBP has been proven to retain its functionality in the absence of the rest of the adhesin.
References
1) Guo, S., Stevens, C., Vance, T., Olijve, L., Graham, L., Campbell, R., . . . Davies, P. (2017). Structure of a 1.5-MDa adhesin that binds its Antarctic bacterium to diatoms and ice. Science Advances, 1-10.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]


