Difference between revisions of "Part:BBa K2812006"
| Line 23: | Line 23: | ||
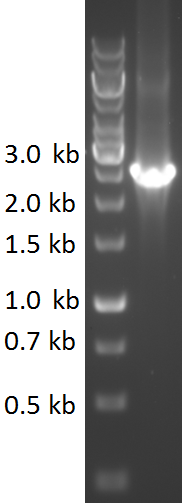
TU-Eindhoven 2018 has characterized the biobrick <partinfo>BBa_K2812006</partinfo> at both the DNA and the protein level. First, the pyocin S5-thrombin linker-HlyA-His-tag construct was synthesized by IDT and subsequently double digested and assembled into the digested linearized pSB1C3 backbone via ligation. The ligated construct was successfully transformed into ''E. coli'' NovaBlue, followed by a colony PCR using the VF2 and VR primers to investigate if the correct length has been inserted in the vector. The mixture was ran on a 1% agarose gel as can be seen in figure 1. The observed length of the brightest band corresponds with the expected length of 1771 basepairs, confirming that the desired construct has been succesfully ligated in pSB1C3 and subsequently transformed in ''E. coli'' NovaBlue. Next, the colonies with the correct insert were cultured in LB before a plasmid purification by a miniprep. The isolated plasmid DNA was sent for Sanger sequencing and the sequence could be confirmed. | TU-Eindhoven 2018 has characterized the biobrick <partinfo>BBa_K2812006</partinfo> at both the DNA and the protein level. First, the pyocin S5-thrombin linker-HlyA-His-tag construct was synthesized by IDT and subsequently double digested and assembled into the digested linearized pSB1C3 backbone via ligation. The ligated construct was successfully transformed into ''E. coli'' NovaBlue, followed by a colony PCR using the VF2 and VR primers to investigate if the correct length has been inserted in the vector. The mixture was ran on a 1% agarose gel as can be seen in figure 1. The observed length of the brightest band corresponds with the expected length of 1771 basepairs, confirming that the desired construct has been succesfully ligated in pSB1C3 and subsequently transformed in ''E. coli'' NovaBlue. Next, the colonies with the correct insert were cultured in LB before a plasmid purification by a miniprep. The isolated plasmid DNA was sent for Sanger sequencing and the sequence could be confirmed. | ||
| + | ====Sources==== | ||
| + | 1) Tossavainen, H., Raulinaitis, V., Kauppinen, L., Pentikäinen, U., Maaheimo, H., & Permi, P. (2018). Structural and Functional Insights Into Lysostaphin–Substrate Interaction. ''Front Mol Biosci''. | ||
| + | |||
| + | 2) Bastos, M. d., Coutinho, B. G., & Coelho, M. L. (2010). Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications. Pharmaceuticals (Basel) , 1139–1161. | ||
| + | |||
| + | 3) Thomas, S., Holland, I. B., & Schmitt, L. (2014). The Type 1 secretion pathway — The hemolysin system and beyond. Molecular Cell Research, 1629-1641. | ||
| + | |||
| + | 4) Gray, L., Baker, K., Kenny, B., Mackman, N., Haigh, R., & Holland, I. (1989). A novel C-terminal signal sequence targets ''Escherichia coli'' haemolysin directly to the medium. J Cell Sci Suppl., 45-57. | ||
| + | |||
| + | 5) Wandersman, C., & Delepelaire, P. (1990 ). TolC, an ''Escherichia coli'' outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A., 4776-4780. | ||
<!-- --> | <!-- --> | ||
Revision as of 23:46, 16 October 2018
Coding sequence for Pyocin S5 with HlyA and His6-tag
Coding sequence for Pyocin S5, which is a bacteriocin produced by a specific strain of P. aeruginosa and is effective against other strains of P. aeruginosa. The sequence encodes for an effector component that consists of a receptor binding domain, a translocation domain and a killing domain. Furthermore it contains an immunity component which gives immunity against the bacteriocin. The HlyA tag is a secretion signal peptide which is used for secretion via the Type 1 secretion pathway of gram-negative bacteria. In order to facilitate protein purification, an N-terminal HIS-tag is present.
Usage and Biology
Pyocin S5
The working mechanism of pyocin S5 involves making the target cell membrane permeable, causing leakage of intracellular materials. It has been demonstrated that is has antimicrobial activity against seven P. aeruginosa strains (DWW3, In3, In4, In7, In8, InA, and InB). P. aeruginosa PAO1, E. coli (BL21 (DE3), W3110, and ATCC15597) and S. aureus ATCC6538 were insensitive to pyocin S5. We as iGem Eindhoven 2018 aimed to use this biobrick for prevention of wound infections.
HlyA
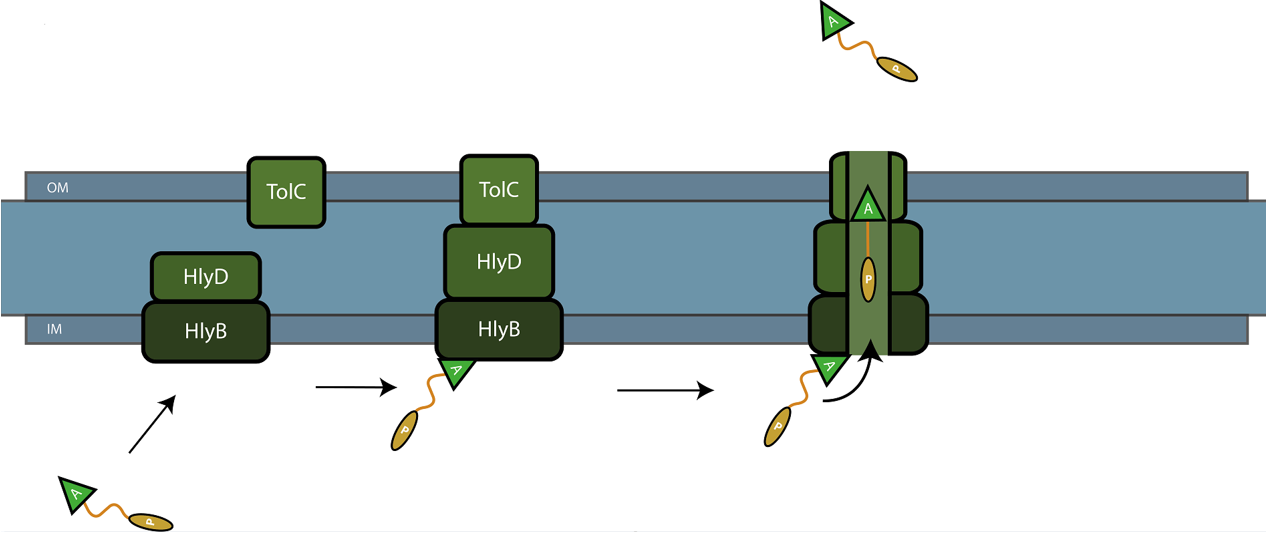
The C-terminal sequence of Hemolysin A (residues 807-1024 of the E. coli HlyA gene) functions as a non-cleavable signal peptide for protein translocation via the Type I secretion pathway of Gram-negative bacteria. Here, HlyA is fused to the pyocin S5 for its secretion from E. coli BL21 (DE3) cells. In Type I secretion, single step transport of the target protein occurs from the cytoplasm to the extracellular environment. The HlyA is fused to the C-terminus of the target protein.3 It is a 23-kDa signal sequence that targets proteins for secretion via Type I secretion pathway. HlyA is secreted into the medium in a TolC and HlyB/D-dependent manner, in the presence of CaCl2.4 It is recognized by the membrane translocation complex composed of HlyB and HlyD, which together with the TolC protein, will form a pore through the membrane.5 This will lead to the secretion of HlyA-containing fusion proteins. Figure 1 illustrates the steps involved in the type I secretion of pyocin S5 as an HlyA fusion protein.
Thrombin linker & His-tag
The pyocin S5 and HlyA are linked via a thrombin linker. It is a short peptide sequence which can be cleaved by the enzyme thrombin, resulting in the removal of the HlyA domain. This avoids interference with the functionality of pyocin S5. C-terminal to the HlyA domain, a His-tag (6x repeated amino acid histidine) is attached. The His-tag can be used for detection of the fusion protein by e.g. Western Blot, but also for protein purification purposes as it facilitates binding to a nickel affinity column.
Fusion protein
The combination of all components described above, lead to a construct that can be (continuously) secreted by E. coli if the HlyB/D coding sequences have been co-transformed. Running the medium containing the fusion protein over a nickel affinity column and subsequent thrombin linker cleavage allows for the easy isolation of purified pyocin S5.
Experimental Characterisation by TU-Eindhoven (2018)
Cloning
TU-Eindhoven 2018 has characterized the biobrick BBa_K2812006 at both the DNA and the protein level. First, the pyocin S5-thrombin linker-HlyA-His-tag construct was synthesized by IDT and subsequently double digested and assembled into the digested linearized pSB1C3 backbone via ligation. The ligated construct was successfully transformed into E. coli NovaBlue, followed by a colony PCR using the VF2 and VR primers to investigate if the correct length has been inserted in the vector. The mixture was ran on a 1% agarose gel as can be seen in figure 1. The observed length of the brightest band corresponds with the expected length of 1771 basepairs, confirming that the desired construct has been succesfully ligated in pSB1C3 and subsequently transformed in E. coli NovaBlue. Next, the colonies with the correct insert were cultured in LB before a plasmid purification by a miniprep. The isolated plasmid DNA was sent for Sanger sequencing and the sequence could be confirmed.
Sources
1) Tossavainen, H., Raulinaitis, V., Kauppinen, L., Pentikäinen, U., Maaheimo, H., & Permi, P. (2018). Structural and Functional Insights Into Lysostaphin–Substrate Interaction. Front Mol Biosci.
2) Bastos, M. d., Coutinho, B. G., & Coelho, M. L. (2010). Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications. Pharmaceuticals (Basel) , 1139–1161.
3) Thomas, S., Holland, I. B., & Schmitt, L. (2014). The Type 1 secretion pathway — The hemolysin system and beyond. Molecular Cell Research, 1629-1641.
4) Gray, L., Baker, K., Kenny, B., Mackman, N., Haigh, R., & Holland, I. (1989). A novel C-terminal signal sequence targets Escherichia coli haemolysin directly to the medium. J Cell Sci Suppl., 45-57.
5) Wandersman, C., & Delepelaire, P. (1990 ). TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A., 4776-4780.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 137
Illegal BglII site found at 425
Illegal BglII site found at 2110 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 1200