Difference between revisions of "Part:BBa K2812004"
m |
|||
| Line 3: | Line 3: | ||
<partinfo>BBa_K2812004 short</partinfo> | <partinfo>BBa_K2812004 short</partinfo> | ||
| − | + | The biobrick contains the coding domain for truncated lysostaphin. Lysostaphin targets the cell wall peptidoglycan found in certain Staphylococci by cleaving its cross-linking pentaglycine bridges. Among others, it is effective for degrading S. aureus biofilms. TU Eindhoven 2018 used lysostaphin to kill Staphylococcus aureus for preventing wound infections. | |
| + | |||
| + | The encoding part of the lysostaphin has been derived from BBa_K748002, made by iGEM Harbin 2012 and is also used by iGEM Stockholm 2016. iGEM Eindhoven 2018 codon optimized this lysostaphin construct. The Hemolysin A (HlyA) signal peptide that is fused to the lysostaphin is involved in the targeting of proteins for secretion via the Type I secretion pathway of gram-negative bacteria. In contrast to the Type II secretion system, single step transport of the target protein occurs from the cytoplasm to the extracellular environment. The HlyA should be fused to the C-terminus of lysostaphin, unlike Type II signal peptides, which should be appended to the N-terminus of the target protein. | ||
| + | |||
| + | The lysostaphin and HlyA are linked via a thrombin linker. This linker can be cleaved of the lysostaphin without leaving behind any additional amino acids, avoiding interference with the functionality of lysostaphin. Finally, a His-tag has been added C-terminal to the HlyA domain, allowing easy purification of pure after isolation. If co-transformed with HlyB/D coding sequences, the construct can be secreted after which isolation from the medium is straightforward. | ||
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | ====Lysostaphin==== | ||
| + | Lysostaphin is an antimicrobial agent produced by Staphylococcus simulans. Lysostaphin belongs to the major class of antimicrobial proteins and peptides known as bacteriocins. Bacteriocins are proteins or peptides produced by bacteria, displaying a bactericidal activity against other subpopulations of bacteria. S. simulans has a protective immune system against lysostaphin, protecting itself from autolysis. | ||
| + | |||
| + | Through cell-wall degradation of the target, lysostaphin has the ability to kill several Staphylococci strains. It owes its cell-wall degradation activity to the endopeptidase activity on pentaglycine cross-bridges in the peptidoglycan layer. Specifically cleavage between the third and fourth glycine residue leads to the lysis of the peptidoglycan layer and death of the bacteria. Resistance of other staphylococcal species to lysostaphin is due to the higher amount of serine residues instead of the glycines. | ||
| + | |||
| + | ====HlyA==== | ||
| + | Escherichia coli haemolysin A (HlyA), which is fused to the lysostaphin, is a signal peptide which targets proteins for secretion via Type I secretion pathway. HlyA is secreted into the medium in a TolC and HlyB/D-dependent manner. In contrast to normal cellular export machineries which transport proteins to the outer membrane or periplasm, secretion is not dependent on both SecA and an N-terminal signal protein. The HlyA signal sequence is attached to the C-terminus of the protein. It is recognized by the membrane translocation complex composed of HlyB and HlyD, which together with the TolC protein, will form a pore through the membrane. This will lead to the secretion of HlyA and the protein fused to HlyA. | ||
| + | |||
| + | The thrombin linker which links the lysostaphin to the HlyA, is a short peptide sequence which is recognised by the enzyme thrombin, which cleaves this linker. In this way, separation of the lysostaphin and HlyA can be controlled. | ||
| + | |||
| + | To the fusion protein, a His-tag (6x repeated amino acid Histidine) is attached. The His-tag is generally used for both detection and purification of proteins. | ||
| + | |||
| + | |||
| + | ==Experimental Characterisation by TU Eindhoven (2018)== | ||
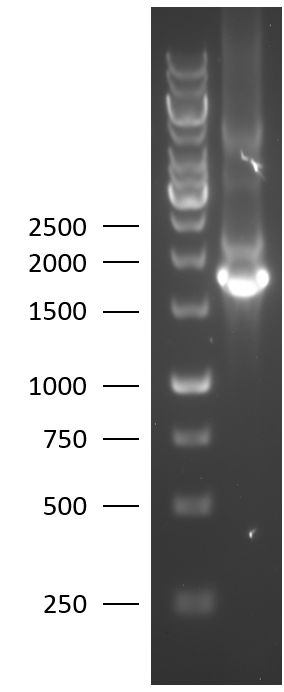
| + | [[File:BBa_K2812004_Agarose_gel.png|125px|thumb|right|1% agarose gel of the colony PCR of the Lysostaphin-HlyA biobrick.]] | ||
| + | ===Cloning=== | ||
| + | TU Eindhoven 2018 has characterized the biobrick at both the DNA and the protein level. First, the lysostaphin-thrombin linker-HlyA-His tag construct was synthesized by IDT and subsequently double digested and assembled into the digested linearized pSB1C3 backbone via ligation. The ligated construct was succesfully transformed into ''E. coli'' Novablue, followed by a colony PCR using the VF2 and VR primers to investigate if the correct length has been inserted in the vector. The mixture was ran on a 1% agarose gel as can be seen on the right. The observed length of the brightest band corresponds with the expected length of X basepairs, confirming that the desired construct has been succesfully ligated in pSB1C3 and subsequently transformed in ''E. coli'' Novablue. Next, the colonies with the correct insert were cultured followed by a miniprep. The isolated plasmid DNA was sent for Sanger sequencing and the sequence could be confirmed. | ||
| + | |||
| + | ===Protein Expression=== | ||
| + | After the succesful characterisation of the biobrick at the DNA level, protein expression experiments were performed. To allow expression of the construct, it was assembled using the T7 promotor <partinfo>BBa_K748002</partinfo> | ||
<!-- --> | <!-- --> | ||
Revision as of 09:05, 15 October 2018
Coding sequence for trunctated Lysostaphin fused to His-tagged HlyA
The biobrick contains the coding domain for truncated lysostaphin. Lysostaphin targets the cell wall peptidoglycan found in certain Staphylococci by cleaving its cross-linking pentaglycine bridges. Among others, it is effective for degrading S. aureus biofilms. TU Eindhoven 2018 used lysostaphin to kill Staphylococcus aureus for preventing wound infections.
The encoding part of the lysostaphin has been derived from BBa_K748002, made by iGEM Harbin 2012 and is also used by iGEM Stockholm 2016. iGEM Eindhoven 2018 codon optimized this lysostaphin construct. The Hemolysin A (HlyA) signal peptide that is fused to the lysostaphin is involved in the targeting of proteins for secretion via the Type I secretion pathway of gram-negative bacteria. In contrast to the Type II secretion system, single step transport of the target protein occurs from the cytoplasm to the extracellular environment. The HlyA should be fused to the C-terminus of lysostaphin, unlike Type II signal peptides, which should be appended to the N-terminus of the target protein.
The lysostaphin and HlyA are linked via a thrombin linker. This linker can be cleaved of the lysostaphin without leaving behind any additional amino acids, avoiding interference with the functionality of lysostaphin. Finally, a His-tag has been added C-terminal to the HlyA domain, allowing easy purification of pure after isolation. If co-transformed with HlyB/D coding sequences, the construct can be secreted after which isolation from the medium is straightforward.
Usage and Biology
Lysostaphin
Lysostaphin is an antimicrobial agent produced by Staphylococcus simulans. Lysostaphin belongs to the major class of antimicrobial proteins and peptides known as bacteriocins. Bacteriocins are proteins or peptides produced by bacteria, displaying a bactericidal activity against other subpopulations of bacteria. S. simulans has a protective immune system against lysostaphin, protecting itself from autolysis.
Through cell-wall degradation of the target, lysostaphin has the ability to kill several Staphylococci strains. It owes its cell-wall degradation activity to the endopeptidase activity on pentaglycine cross-bridges in the peptidoglycan layer. Specifically cleavage between the third and fourth glycine residue leads to the lysis of the peptidoglycan layer and death of the bacteria. Resistance of other staphylococcal species to lysostaphin is due to the higher amount of serine residues instead of the glycines.
HlyA
Escherichia coli haemolysin A (HlyA), which is fused to the lysostaphin, is a signal peptide which targets proteins for secretion via Type I secretion pathway. HlyA is secreted into the medium in a TolC and HlyB/D-dependent manner. In contrast to normal cellular export machineries which transport proteins to the outer membrane or periplasm, secretion is not dependent on both SecA and an N-terminal signal protein. The HlyA signal sequence is attached to the C-terminus of the protein. It is recognized by the membrane translocation complex composed of HlyB and HlyD, which together with the TolC protein, will form a pore through the membrane. This will lead to the secretion of HlyA and the protein fused to HlyA.
The thrombin linker which links the lysostaphin to the HlyA, is a short peptide sequence which is recognised by the enzyme thrombin, which cleaves this linker. In this way, separation of the lysostaphin and HlyA can be controlled.
To the fusion protein, a His-tag (6x repeated amino acid Histidine) is attached. The His-tag is generally used for both detection and purification of proteins.
Experimental Characterisation by TU Eindhoven (2018)
Cloning
TU Eindhoven 2018 has characterized the biobrick at both the DNA and the protein level. First, the lysostaphin-thrombin linker-HlyA-His tag construct was synthesized by IDT and subsequently double digested and assembled into the digested linearized pSB1C3 backbone via ligation. The ligated construct was succesfully transformed into E. coli Novablue, followed by a colony PCR using the VF2 and VR primers to investigate if the correct length has been inserted in the vector. The mixture was ran on a 1% agarose gel as can be seen on the right. The observed length of the brightest band corresponds with the expected length of X basepairs, confirming that the desired construct has been succesfully ligated in pSB1C3 and subsequently transformed in E. coli Novablue. Next, the colonies with the correct insert were cultured followed by a miniprep. The isolated plasmid DNA was sent for Sanger sequencing and the sequence could be confirmed.
Protein Expression
After the succesful characterisation of the biobrick at the DNA level, protein expression experiments were performed. To allow expression of the construct, it was assembled using the T7 promotor BBa_K748002
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1351
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 126
- 1000COMPATIBLE WITH RFC[1000]