Difference between revisions of "Part:BBa K2549013"
(→Usage and Biology) |
(→Applications) |
||
| Line 16: | Line 16: | ||
[[File:TEV1.png|none|400px|thumb|Waugh DS wrote: ''TEV protease sequence specificity is far more stringent than that of factor Xa, thrombin, or enterokinase. It is a very useful reagent for cleaving fusion proteins. TEV protease recognizes a linear epitope of the general form E-Xaa-Xaa-Y-Xaa-Q-(G/S), with cleavage occurring between Q and G or Q and S.'']] | [[File:TEV1.png|none|400px|thumb|Waugh DS wrote: ''TEV protease sequence specificity is far more stringent than that of factor Xa, thrombin, or enterokinase. It is a very useful reagent for cleaving fusion proteins. TEV protease recognizes a linear epitope of the general form E-Xaa-Xaa-Y-Xaa-Q-(G/S), with cleavage occurring between Q and G or Q and S.'']] | ||
Please refer to [[Media:Tev-faq-7pages.pdf]] for more details on TEV protease. | Please refer to [[Media:Tev-faq-7pages.pdf]] for more details on TEV protease. | ||
| + | |||
| + | ==== TEV in Tango Assay for GPCRs ==== | ||
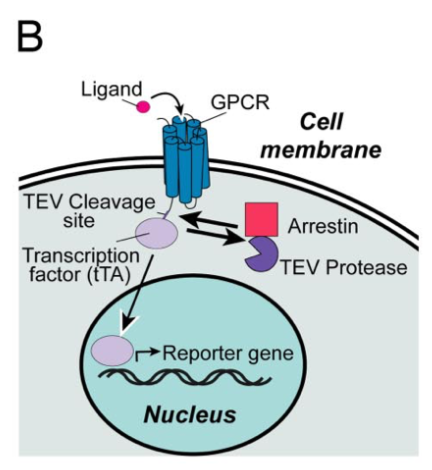
| + | Barnea G et al 2008<ref>The genetic design of signaling cascades to record receptor activation. Barnea G, Strapps W, Herrada G, ..., Axel R, Lee KJ. Proc Natl Acad Sci U S A, 2008 Jan;105(1):64-9 PMID: 18165312; DOI: 10.1073/pnas.0710487105</ref> wrote: ''We have developed an experimental strategy to monitor protein interactions in a cell with a high degree of selectivity and sensitivity. A transcription factor is tethered to a membrane-bound receptor with a linker that contains a cleavage site for a specific protease. Activation of the receptor recruits a signaling protein fused to the protease that then cleaves and releases the transcription factor to activate reporter genes in the nucleus. This strategy converts a transient interaction into a stable and amplifiable reporter gene signal to record the activation of a receptor without interference from endogenous signaling pathways. We have developed this assay for three classes of receptors: G protein-coupled receptors, receptor tyrosine kinases, and steroid hormone receptors. Finally, we use the assay to identify a ligand for the orphan receptor GPR1, suggesting a role for this receptor in the regulation of inflammation.'' | ||
| + | |||
| + | [[File:TEV3.png|none|200px|thumb|Barnea G et al stated: ''Schematic of the Tango assay method to monitor GPCR–arrestin interactions. Ligand binding to the target receptor stimulates recruitment of the arrestin-TEV protease fusion, triggering the release of the tethered transcription factor tTA. Free tTA then enters the nucleus and stimulates reporter gene activity.'']] | ||
| + | |||
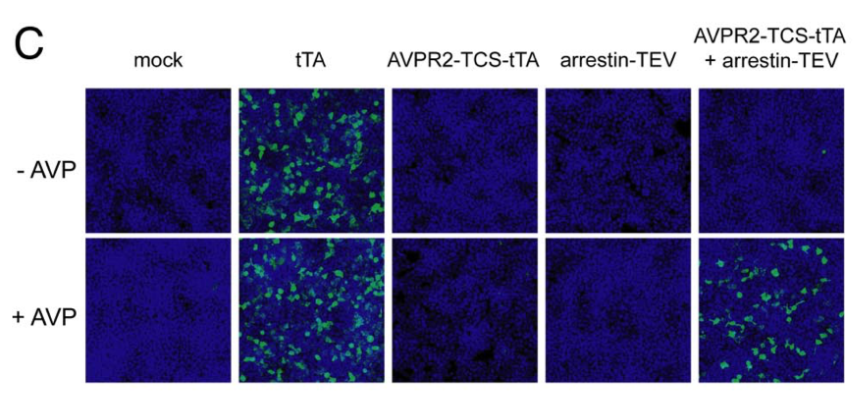
| + | [[File:TEV4.png|none|400px|thumb|Barnea G et al stated: ''Tango assay for the arginine vasopressin receptor AVPR2. HTL cells (an HEK293T-derived cell line containing a stably integrated tTA-dependent luciferase reporter) were transiently transfected with expression plasmids encoding the arginine vasopressin receptor 2 (AVPR2)-TEV cleavage site-tTA (AVPR2-TCS-tTA) and β-arrestin2-TEV protease (Arrestin-TEV) fusion proteins, as indicated. Control cells were transfected either with a tTA expression plasmid (tTA) or with empty parental expression plasmid (mock). Luciferase expression was detected by immunostaining with anti-luciferase (green), and cell nuclei were visualized with the DNA dye TOTO-3 (blue). Western blot analysis confirmed that receptor and arrestin components were expressed at similar levels in each transfection.'']] | ||
| + | |||
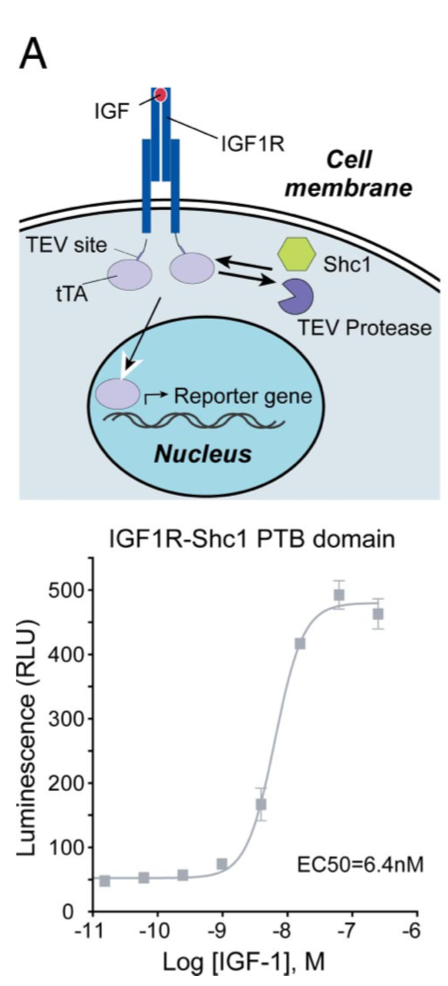
| + | [[File:TEV5.png|none|200px|thumb|Barnea G et al stated: ''Receptor tyrosine kinase signaling. HTL cells were transfected with an IGF-1 receptor (IGF1R)–TCS-tTA fusion construct and a Shc1 PTB domain-TEV protease fusion plasmid. Luciferase activity in this IGF1R–Shc1 interaction assay was stimulated by IGF-1. All error bars represent SD (n = four measurements).'']] | ||
| + | |||
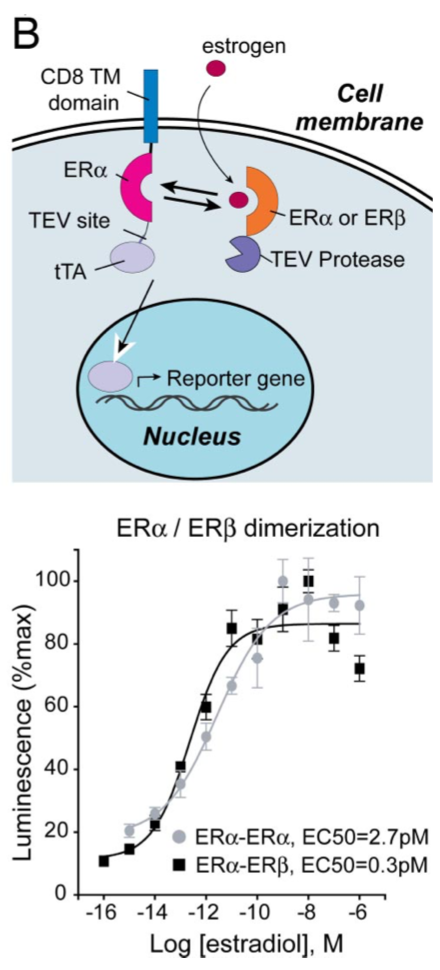
| + | [[File:TEV6.png|none|200px|thumb|Barnea G et al stated: ''Ligand-induced homo- and heterodimerization of estrogen receptors (ERs). HTL cells were transfected with a CD8-ERα-TCS-tTA fusion and either an ERα- or ERβ-TEV protease fusion to measure ERα homodimerization (light circles) or ERα/ERβ heterodimerization (dark squares) as illustrated. Responses were normalized to the maximal response for each receptor. All error bars represent SD (n = four measurements).'']] | ||
| + | |||
Note: [[Part:BBa_K2549013]], [[Part:BBa_K2549014]] and [[Part:BBa_K2549015]] have the same Applications section. | Note: [[Part:BBa_K2549013]], [[Part:BBa_K2549014]] and [[Part:BBa_K2549015]] have the same Applications section. | ||
Revision as of 14:06, 7 October 2018
TEV protease
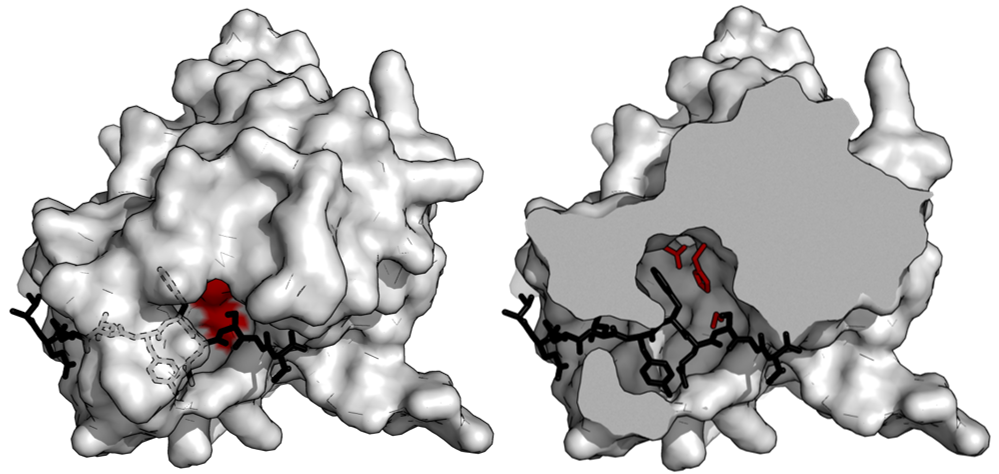
This part is a mutant TEV (tobacco etch virus) protease. The residue 219 of the wild-type TEV protease is mutated from serine to valine (S219V) to remove autoinactivation and perform a more efficient cleavage[1]. TEV protease is one of the best-characterized enzymes of the viral proteases which have more stringent sequence specificity. We used it to build our IMPLY logic gates. It can also be utilized by other iGEM teams to create their own engineered fusion proteins cleavage system.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 316
Applications
The main use of TEV protease[2] is for removing affinity tags from purified recombinant fusion proteins. The reason for that is due to the high sequence specificity. This specificity allows for the controlled cleavage of proteins when the preference sequence is inserted into flexible loops. It also makes it relatively non-toxic in vivo as the recognized sequence scarcely occurs in proteins.
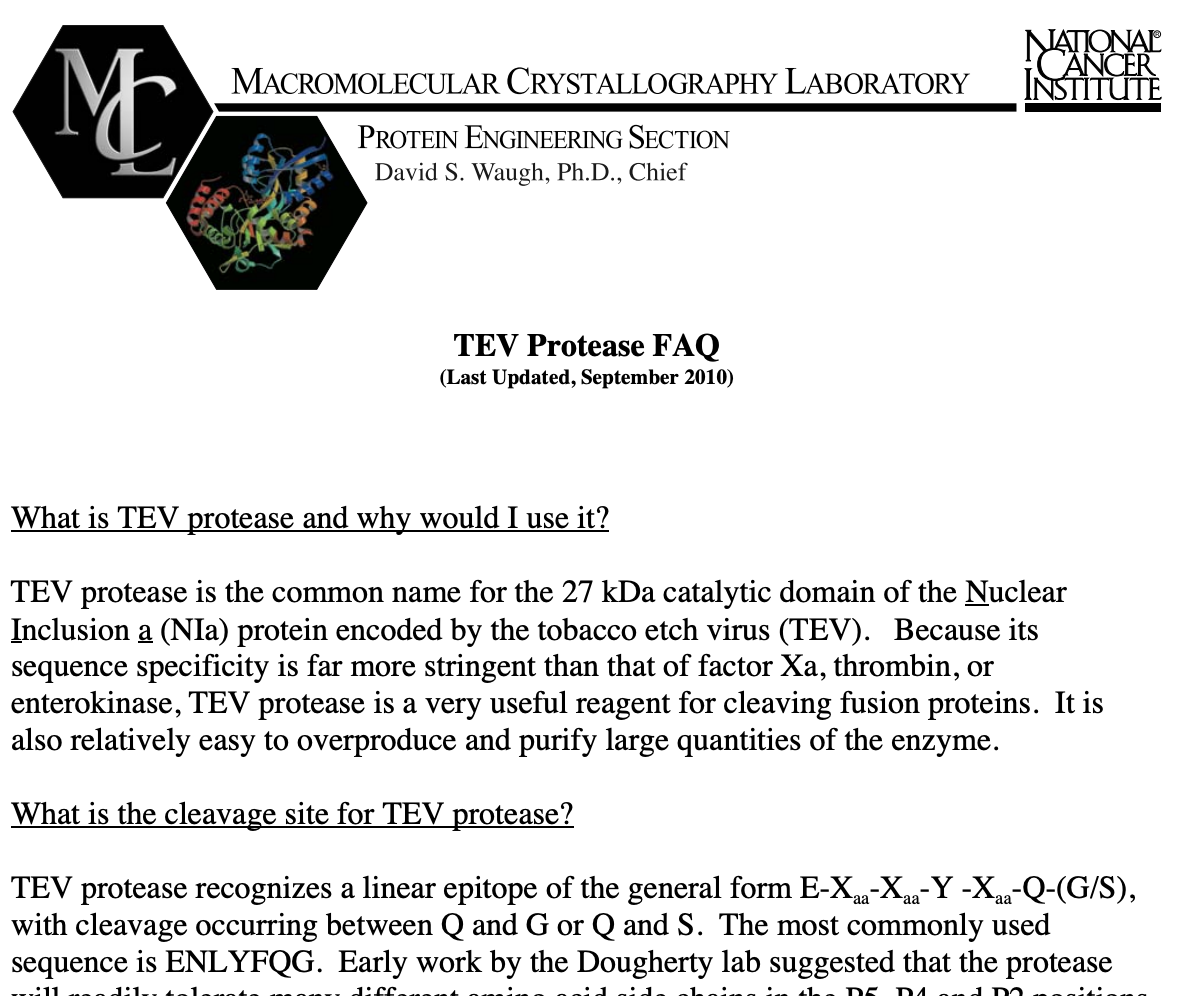
Please refer to Media:Tev-faq-7pages.pdf for more details on TEV protease.
TEV in Tango Assay for GPCRs
Barnea G et al 2008[3] wrote: We have developed an experimental strategy to monitor protein interactions in a cell with a high degree of selectivity and sensitivity. A transcription factor is tethered to a membrane-bound receptor with a linker that contains a cleavage site for a specific protease. Activation of the receptor recruits a signaling protein fused to the protease that then cleaves and releases the transcription factor to activate reporter genes in the nucleus. This strategy converts a transient interaction into a stable and amplifiable reporter gene signal to record the activation of a receptor without interference from endogenous signaling pathways. We have developed this assay for three classes of receptors: G protein-coupled receptors, receptor tyrosine kinases, and steroid hormone receptors. Finally, we use the assay to identify a ligand for the orphan receptor GPR1, suggesting a role for this receptor in the regulation of inflammation.
Note: Part:BBa_K2549013, Part:BBa_K2549014 and Part:BBa_K2549015 have the same Applications section.
References
- ↑ Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Kapust RB, Tözsér J, Fox JD, ..., Copeland TD, Waugh DS. Protein Eng, 2001 Dec;14(12):993-1000 PMID: 11809930
- ↑ https://en.wikipedia.org/wiki/TEV_protease
- ↑ The genetic design of signaling cascades to record receptor activation. Barnea G, Strapps W, Herrada G, ..., Axel R, Lee KJ. Proc Natl Acad Sci U S A, 2008 Jan;105(1):64-9 PMID: 18165312; DOI: 10.1073/pnas.0710487105