Difference between revisions of "Part:BBa R0082"
| Line 26: | Line 26: | ||
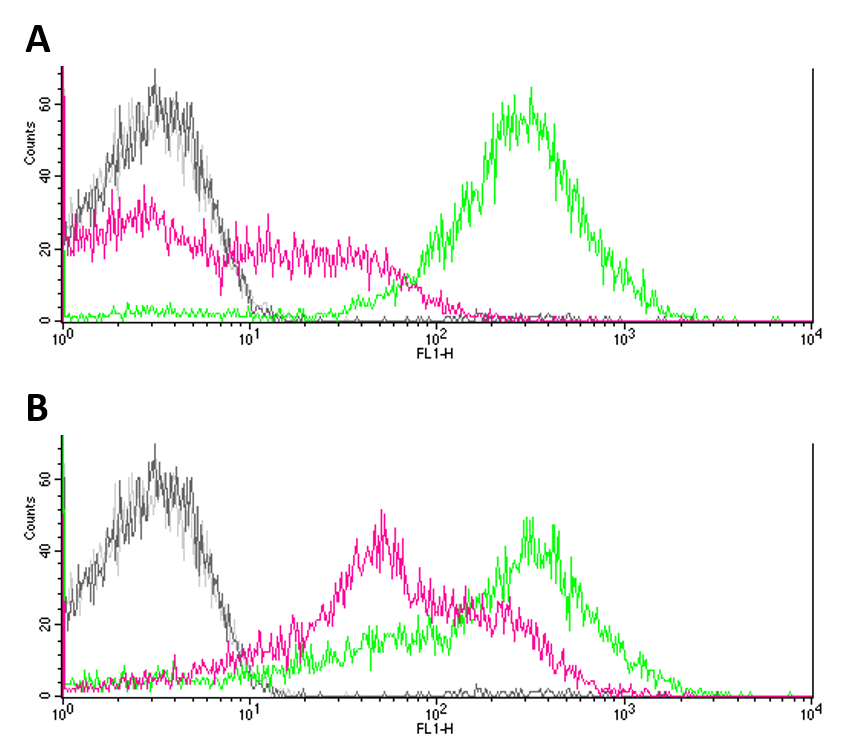
Negative controls (JW3367-3 and DH5a without reporter systems) showed only few to no fluorescence (Fig.1 A, B). GFP expression (Fig. 1 A) proved to be significantly higher in DH5a (green) cells as compared to EnvZ deficient JW3367-3 cells (pink). Although not as pronounced, this difference was also observable for eYFP expression in the respective cell lines (fig. 1 B). Differences in the expression of fluorescence reporters were confirmed by KS tests (GFP: p <= 0.001, eYFP: p <= 0.001). </p> | Negative controls (JW3367-3 and DH5a without reporter systems) showed only few to no fluorescence (Fig.1 A, B). GFP expression (Fig. 1 A) proved to be significantly higher in DH5a (green) cells as compared to EnvZ deficient JW3367-3 cells (pink). Although not as pronounced, this difference was also observable for eYFP expression in the respective cell lines (fig. 1 B). Differences in the expression of fluorescence reporters were confirmed by KS tests (GFP: p <= 0.001, eYFP: p <= 0.001). </p> | ||
[[Image:BBa_R0082-FACS_noise.png|500px]] | [[Image:BBa_R0082-FACS_noise.png|500px]] | ||
| + | |||
| + | ===Leaky Expression by the OmpR-Regulated Promoter on Different Vectors=== | ||
| + | <b>The leaky expression by the OmpR-regulated promoter is reduced when cloned into a low copy vector compared to a high copy vector.</b><br> | ||
| + | The expression properties of the OmpR-regulated promoter were investigated using a reporter system containing RFP under control of the OmpR-regulated promoter, <partinfo>BBa_M30011</partinfo>, was cloned into <i>E. coli</i> strain SØ928 ΔOmpR, lacking the OmpR transcription factor, on a high copy vector. By using a ΔOmpR strain, the background generated by stimulation of the intrinsic OmpR system is removed, and the strain functions as a negative control. <br> | ||
| + | RFP expression was assessed by fluorescence microscopy using an Olympus IX83 with a photometrics prime camera, with exposure time for RFP at 200 ms. | ||
| + | Assessing the RFP expression by fluorescence microscopy, it was discovered that the OmpR-regulated promoter mediated gene expression even in the absence of its transcription factor, see Figure 1. This observation was confirmed by going through the literature [https://www.ncbi.nlm.nih.gov/pubmed/16306980 Levskaya A] | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
Revision as of 02:24, 2 November 2017
Promoter (OmpR, positive)
Positively regulated, OmpR-controlled promoter. This promoter is taken from the upstream region of ompC. Phosphorylated OmpR binds to the three operator sites and activates transcription.
Usage and Biology
In nature, this promoter is upstream of the ompC porin gene. The regulation of ompC is determined by the EnvZ-OmpR osmosensing machinery. EnvZ phosphorylates OmpR to OmpR-P. At high osmolarity, EnvZ is more active, creating more OmpR-P. OmpR-P then binds to the low-affinity OmpR operator sites upstream of ompC.
Contribution: Hamburg 2016
Group: Hamburg / Authors: Kai Pohl, Daniel Wedemeyer
Summary: We analysed the noise produced by BBa_R0082 by flow cytometry using a ΔEnvZ strain.
In two approaches, we ligated BBa_R0082 (OmpR, positive promoter) with BBa_E0240 (GFP) and BBa_E0430 (eYFP) into pSB1C3 transformed both ΔEnvZ (JW3367-3) and wt (DH5a) E. coli strains with them. Constructs were validated by sequencing. We submitted the constructs as: BBa_K1909013 (Omp/GFP) and BBa_K1909014 (Omp/eYFP) to the registry.
Experiments
To determine the noise of the OmpR promoter, we measured the expression of our Fluorescence reporters by flow cytometry, comparing the ΔEnvZ (JW3367-3) to wt (DH5a) E. coli strain. Furthermore, we used JW3367-3 and DH5a strains without our reporter system as negative controls. Cells were incubated in LB medium to OD600 = 0.2, washed using 100 mM MgCl2 and diluted 1:100 prior to measuring.
Results
Negative controls (JW3367-3 and DH5a without reporter systems) showed only few to no fluorescence (Fig.1 A, B). GFP expression (Fig. 1 A) proved to be significantly higher in DH5a (green) cells as compared to EnvZ deficient JW3367-3 cells (pink). Although not as pronounced, this difference was also observable for eYFP expression in the respective cell lines (fig. 1 B). Differences in the expression of fluorescence reporters were confirmed by KS tests (GFP: p <= 0.001, eYFP: p <= 0.001).
Leaky Expression by the OmpR-Regulated Promoter on Different Vectors
The leaky expression by the OmpR-regulated promoter is reduced when cloned into a low copy vector compared to a high copy vector.
The expression properties of the OmpR-regulated promoter were investigated using a reporter system containing RFP under control of the OmpR-regulated promoter, BBa_M30011, was cloned into E. coli strain SØ928 ΔOmpR, lacking the OmpR transcription factor, on a high copy vector. By using a ΔOmpR strain, the background generated by stimulation of the intrinsic OmpR system is removed, and the strain functions as a negative control.
RFP expression was assessed by fluorescence microscopy using an Olympus IX83 with a photometrics prime camera, with exposure time for RFP at 200 ms.
Assessing the RFP expression by fluorescence microscopy, it was discovered that the OmpR-regulated promoter mediated gene expression even in the absence of its transcription factor, see Figure 1. This observation was confirmed by going through the literature Levskaya A
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]