Difference between revisions of "Part:BBa K2457002"
Jocomodaro (Talk | contribs) (→Usage and Biology) |
Jocomodaro (Talk | contribs) (→Design) |
||
| Line 15: | Line 15: | ||
===Design=== | ===Design=== | ||
| + | <b>Synthetic sgRNA structures</b> | ||
| + | <p>Nowadays, the first synthetic sgRNA chimera - which was designed by Jinek <i>et al.</i> in 2012 - is the commonly applied RNA structure in the scientific projects AND in the iGEM devices. It has a shortened duplex (guide: target DNA heteroduplex via 20 Rosalind-Watson-Crick base pair + linker loop + 30bp tracrRNA) compared with the native bacterial CRISPR RNA (42bp crRNA + 89bp tracrRNA), as illustrated in Figure 4.</p> | ||
| + | <p>However, despite sgRNA with a 30-bp tracrRNA tail is the shorter although sgRNA with a 48-nt tracrRNA tail is the minimal region for the Cas9-catalyzed DNA cleavage in vitro (Jinek et al., 2012), sgRNAs with extended tracrRNA tails, sgRNA(+67) and sgRNA(+85), dramatically improved the Cas9 cleavage activity in vivo (Hsu et al., 2013). The present structure revealed that sgRNA(+48), sgRNA(+67) and sgRNA(+85) contain stem loop 1, stem loops 1–2 and stem loops 1–3, respectively (Figures 4A, B). These observations indicated that, whereas stem loop 1 is essential for the formation of the functional Cas9–sgRNA complex, stem loops 2 and 3 further support the stable complex formation and enhance the stability of the sgRNA, thus improving the in vivo activity.</p> | ||
===Characterization=== | ===Characterization=== | ||
Revision as of 18:52, 1 November 2017
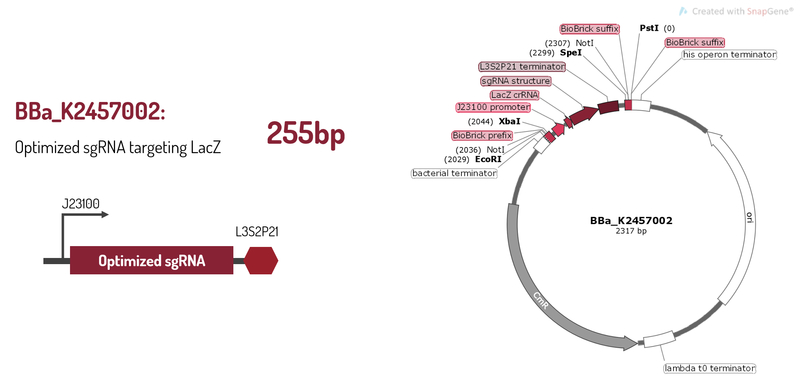
Optimized sgRNA targeting LacZ
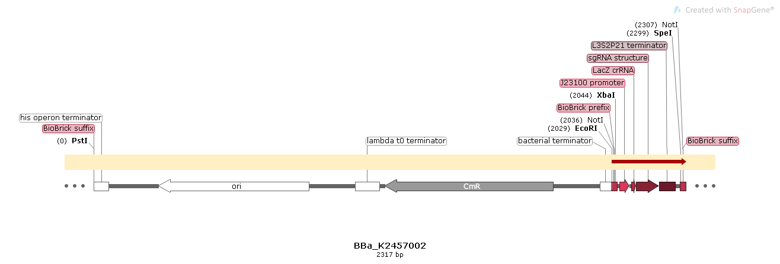
BBa_K2457002 was engineered to be a building block of the standard BioBrick toolbox for bacterial genome: the ([http://2017.igem.org/Team:Amazonas_Brazil pCRISPeasy]). It’s the first BioBrick available on Registry with an improved sgRNA sequence - both crRNA design and tracrRNA secondary structure - to increase the CRISPR-Cas9 editing efficiency. BBa_K2457002 is composed by: the J23100 constitutive promoter sequence + an optimized sgRNA (crRNA with a GG motif at the 3’ end of it’s target-specific sequence from LacZ [1] and tracrRNA with extended duplex length and a mutation at the thymine 4 [2]) + L3S2P21 terminator [3]. It was rationally designed to be standardized AND interchangeable, aiming to provide a BioBrick to attend multiple genome editing purposes. For that matter, It’s easy to engineer new modules aiming to insert information into the genome through RFC10 Assembly.
Figure 1: BBa_K2457002 circuit.
Usage and Biology
Single-guide RNA (sgRNA) is one of the two building blocks of the CRISPR-Cas9 genome editing machinery, which directs the Cas9 protein to introduce double-stranded breaks (DSB) into target genomes sequences.
In nature, after the memory development fase made by Cas proteins, the CRISPR locus is transcribed by RNA polymerase synthesizing the crRNA precursor.The post-transcriptional maturation process begins, which this pre-crRNA through your complementarity of your palindromic repetition, hybridizes itself with a transactivator RNA (tracrRNA), catalyzing the double strand ribonuclease formation RNAses III with the Cas9 protein. This complex cleaves the upstream palindromic repeated hairspin, making smalls hybridized crRNAs with the transactivator RNA (tracrRNA) in a duo composed by crRNA:tracrRNA, carrying a specific protospacer (complementary sequence) from a invasor DNA. This system, demonstrated on Figure 2 below, Deltcheva et al suggested in 2011, having as inspiration the microRNAs by interference RNAs in eukaryotes.
Therefore, the complex crRNA:tracrRNA consists of: a crRNA, with 42 nucleotides, deriving from the pre-crRNA transcribed on locus CRISPR; and a tracrRNA with 89 nucleotides, transcribed upstream to Cas operon. This non-coding RNAs hybridize themselves by Rosalind-Watson-Crick base pairing, composing a more negatively charged structure. Through polar interaction and bases stacking, the crRNA:tracrRNA is adjusted in a positive charged site between REC and NUC lobuses interface originated at Cas9 protein stemming from Streptococcus pyogenes, changing its quaternary structure to a catalytically active ribonucleoprotein complex (RNP), with the ability to cleave DNA target sequences that are complementary to the 20 guide nucleotides presents in crRNA through the HNH and RuvC nuclease domains.
Design
Synthetic sgRNA structures
Nowadays, the first synthetic sgRNA chimera - which was designed by Jinek et al. in 2012 - is the commonly applied RNA structure in the scientific projects AND in the iGEM devices. It has a shortened duplex (guide: target DNA heteroduplex via 20 Rosalind-Watson-Crick base pair + linker loop + 30bp tracrRNA) compared with the native bacterial CRISPR RNA (42bp crRNA + 89bp tracrRNA), as illustrated in Figure 4.
However, despite sgRNA with a 30-bp tracrRNA tail is the shorter although sgRNA with a 48-nt tracrRNA tail is the minimal region for the Cas9-catalyzed DNA cleavage in vitro (Jinek et al., 2012), sgRNAs with extended tracrRNA tails, sgRNA(+67) and sgRNA(+85), dramatically improved the Cas9 cleavage activity in vivo (Hsu et al., 2013). The present structure revealed that sgRNA(+48), sgRNA(+67) and sgRNA(+85) contain stem loop 1, stem loops 1–2 and stem loops 1–3, respectively (Figures 4A, B). These observations indicated that, whereas stem loop 1 is essential for the formation of the functional Cas9–sgRNA complex, stem loops 2 and 3 further support the stable complex formation and enhance the stability of the sgRNA, thus improving the in vivo activity.
Characterization
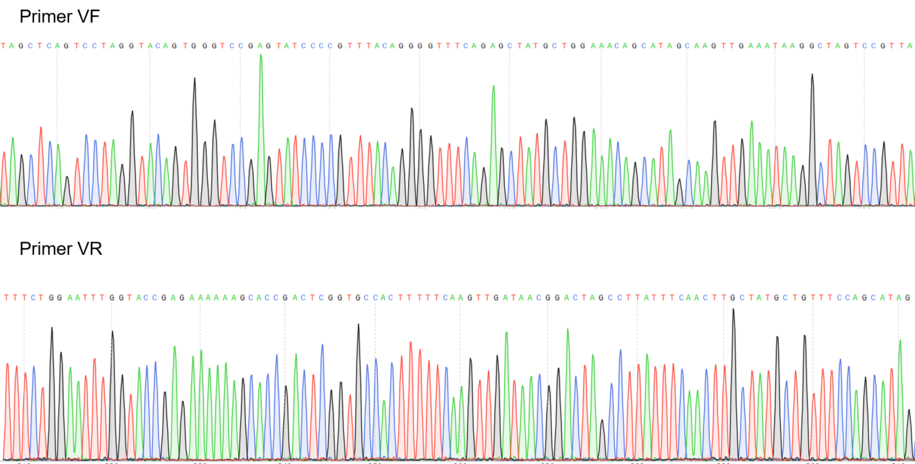 Figure 2: Sequencing electropherogram from BBa_K2457002.
Figure 2: Sequencing electropherogram from BBa_K2457002.
Figure 3: Alignment of the designed sequence and our final construction from BBa_K2457002.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]