Difference between revisions of "Part:BBa K2384011"
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K2384011 short</partinfo> | <partinfo>BBa_K2384011 short</partinfo> | ||
| − | <mf>-Lon is endogenous Lon protease based on the Gram-positive <i>M.florum</i> tmRNA system. | + | <br/><br/> |
| + | <i>mf</i>-Lon is endogenous Lon protease based on the Gram-positive <i>M.florum</i> tmRNA system. | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
Exogenous control of protein biosynthesis through transcriptional and translational regulation has been well established, but robust and tunable control of protein degradation in bacteria remains elusive. | Exogenous control of protein biosynthesis through transcriptional and translational regulation has been well established, but robust and tunable control of protein degradation in bacteria remains elusive. | ||
| − | < | + | <br/><br/> |
<b><i>mf</i>-Lon</b> | <b><i>mf</i>-Lon</b> | ||
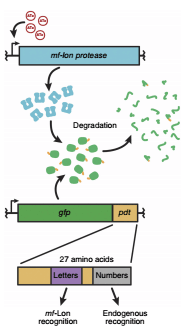
Here we present a synthetic degradation system based on the Gram-positive <i>M.florum</i> tmRNA system that does not rely on host degradation systems and function in a wide range of bacteria. | Here we present a synthetic degradation system based on the Gram-positive <i>M.florum</i> tmRNA system that does not rely on host degradation systems and function in a wide range of bacteria. | ||
| Line 16: | Line 17: | ||
Protein degradation in bacteria occurs in part through the transfer-messenger RNA (tmRNA) system, which uses C-terminal fusion of the ssrA peptide to direct proteins to the endogenous ClpXP and ClpAP proteases for rapid degradation in <i>E. coli</i>. Variants of the | Protein degradation in bacteria occurs in part through the transfer-messenger RNA (tmRNA) system, which uses C-terminal fusion of the ssrA peptide to direct proteins to the endogenous ClpXP and ClpAP proteases for rapid degradation in <i>E. coli</i>. Variants of the | ||
<i>E. coli</i> ssrA tag (ec-ssrA) are commonly used to modify the degradation rate of attached proteins in both bacteria and eukaryotes, but these tags do not provide inducible control of degradation. Recently developed inducible eukaryotic systems rely on degradation machinery not present in bacteria and bacterial systems such as the one developed by Davis et al. require disruption of the endogenous tmRNA system and are therefore not easily transferred to other organisms. | <i>E. coli</i> ssrA tag (ec-ssrA) are commonly used to modify the degradation rate of attached proteins in both bacteria and eukaryotes, but these tags do not provide inducible control of degradation. Recently developed inducible eukaryotic systems rely on degradation machinery not present in bacteria and bacterial systems such as the one developed by Davis et al. require disruption of the endogenous tmRNA system and are therefore not easily transferred to other organisms. | ||
| − | < | + | <br/><br/> |
Toggle switch control of targeted essential protein degradation. Inclusion of the mf-Lon–specific pdt#1 tag on the specified essential gene causes mf-Lon–mediated degradation of the essential protein upon circuit activation. | Toggle switch control of targeted essential protein degradation. Inclusion of the mf-Lon–specific pdt#1 tag on the specified essential gene causes mf-Lon–mediated degradation of the essential protein upon circuit activation. | ||
| + | <br/><br/> | ||
| + | <table><tr> | ||
| + | <th>[[Image:T--FAFU-CHINA-Toggle.png|thumb|400px|'''Figure 2''':Toggle switch. ]]</th> | ||
| + | </tr></table> | ||
| + | |||
Revision as of 13:10, 1 November 2017
mf-Lon
mf-Lon is endogenous Lon protease based on the Gram-positive M.florum tmRNA system.
Usage and Biology
Exogenous control of protein biosynthesis through transcriptional and translational regulation has been well established, but robust and tunable control of protein degradation in bacteria remains elusive.
mf-Lon
Here we present a synthetic degradation system based on the Gram-positive M.florum tmRNA system that does not rely on host degradation systems and function in a wide range of bacteria.
Protein degradation in bacteria occurs in part through the transfer-messenger RNA (tmRNA) system, which uses C-terminal fusion of the ssrA peptide to direct proteins to the endogenous ClpXP and ClpAP proteases for rapid degradation in E. coli. Variants of the
E. coli ssrA tag (ec-ssrA) are commonly used to modify the degradation rate of attached proteins in both bacteria and eukaryotes, but these tags do not provide inducible control of degradation. Recently developed inducible eukaryotic systems rely on degradation machinery not present in bacteria and bacterial systems such as the one developed by Davis et al. require disruption of the endogenous tmRNA system and are therefore not easily transferred to other organisms.
Toggle switch control of targeted essential protein degradation. Inclusion of the mf-Lon–specific pdt#1 tag on the specified essential gene causes mf-Lon–mediated degradation of the essential protein upon circuit activation.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NotI site found at 25
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 2498
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]