Difference between revisions of "Help:2017 Competent Cell Test Kit"
| Line 14: | Line 14: | ||
<td style="vertical-align: top; padding: 10px;"> | <td style="vertical-align: top; padding: 10px;"> | ||
<p>Before using your competent cells in an experiment, use the <b>Competent Cell Test Kit</b> to test the efficiency of your competent cells!</p> | <p>Before using your competent cells in an experiment, use the <b>Competent Cell Test Kit</b> to test the efficiency of your competent cells!</p> | ||
| − | <p>The kit includes three vials of purified plasmid DNA from <a href="https://parts.igem.org/Part:BBa_J04450">BBa_J04450</a> (RFP construct) in plasmid backbone pSB1C3. Each vial contains DNA at a different concentration: 100 pg/µL, 50 pg/µL, 10 pg/µL. Perform transformations with each of these to determine how efficient your competent cells are.</p> | + | <p>The kit includes three vials of purified plasmid DNA from <a href="https://parts.igem.org/Part:BBa_J04450">BBa_J04450</a> (RFP construct) in plasmid backbone pSB1C3. Each vial contains 50 µL of DNA at a different concentration: 100 pg/µL, 50 pg/µL, 10 pg/µL. Perform transformations with each of these to determine how efficient your competent cells are.</p> |
| − | <p>It is important to have efficient competent cells because transformations performed with ligation products usually do not yield as many colonies | + | <p>It is important to have efficient competent cells because transformations performed with ligation products usually do not yield as many colonies due to the low DNA concentration in the ligation mixture. This means that you may see different results doing this test than you will at the end of the 3A Assembly protocol, or any other ligation.</p> |
| + | <p>You can use the following suggested protocol for heat-shock transformation to test your chemically-competent <i>E. coli</i> cells.</p> | ||
</td> | </td> | ||
| Line 26: | Line 27: | ||
*Paper towels | *Paper towels | ||
*Lab marker / Sharpie | *Lab marker / Sharpie | ||
| + | *1.5 mL microcentrifuge tubes | ||
*Container for ice | *Container for ice | ||
*Ice | *Ice | ||
| Line 31: | Line 33: | ||
*'''Competent Cell Test Kit''' | *'''Competent Cell Test Kit''' | ||
*Agar plates with chloramphenicol | *Agar plates with chloramphenicol | ||
| − | *Waterbath (or hot water source and thermometer) | + | *42°C Waterbath (or hot water source and thermometer) |
| − | * | + | *37°C Incubators (oven and shaker) |
*SOC media | *SOC media | ||
| − | *Sterile glass beads or | + | *Sterile glass beads or sterile cell spreader |
*Pipettor | *Pipettor | ||
*Pipette tips | *Pipette tips | ||
| Line 41: | Line 43: | ||
===Protocol=== | ===Protocol=== | ||
''estimated time: 30 minutes active, 1.5 hours incubation'' | ''estimated time: 30 minutes active, 1.5 hours incubation'' | ||
| + | #Clean your working area by wiping down with 70% ethanol. | ||
| + | #Thaw competent cells on ice. Label one 1.5 mL microcentrifuge tubes for each transformation and then pre-chill by placing the tubes on ice. | ||
| + | #*Do triplicates (3 each) of each concentration if possible, so you can calculate an average colony yield. | ||
#Spin down the DNA tubes from the Competent Cell Test Kit/Transformation Efficiency Kit to collect all of the DNA into the bottom of each tube prior to use. A quick spin of 20-30 seconds at 8,000-10,000 rpm will be sufficient. <i>Note:</i> There should be 50 µL of DNA in each tube sent in the Kit. | #Spin down the DNA tubes from the Competent Cell Test Kit/Transformation Efficiency Kit to collect all of the DNA into the bottom of each tube prior to use. A quick spin of 20-30 seconds at 8,000-10,000 rpm will be sufficient. <i>Note:</i> There should be 50 µL of DNA in each tube sent in the Kit. | ||
| − | + | #Pipet 1 µL of DNA into each microcentrifuge tube. | |
| − | #Pipet 1 µL of DNA into each microcentrifuge tube | + | #Pipet 50 µL of competent cells into each tube. Flick the tube gently with your finger to mix. |
| − | #Pipet 50 µL of competent cells into each tube. Flick the tube gently with your finger to mix. Incubate on ice for 30 minutes. Pre-heat waterbath now to 42°C. Otherwise, hot water and an accurate thermometer works, too! | + | #Incubate on ice for 30 minutes. |
| − | #Heat-shock the cells by placing into the waterbath for 45 seconds. Be careful to keep the lids of the tubes above the water level, and keep the ice close by. | + | #*Pre-heat waterbath now to 42°C. Otherwise, hot water and an accurate thermometer works, too! |
| − | #Immediately transfer the tubes back to ice, and incubate on ice for 5 minutes | + | #Heat-shock the cells by placing into the waterbath for 45 seconds (no longer than 1 min). Be careful to keep the lids of the tubes above the water level, and keep the ice close by. |
| − | #Add 950 µL of SOC media per tube, and incubate at 37°C for 1 hours shaking at | + | #Immediately transfer the tubes back to ice, and incubate on ice for 5 minutes. |
| − | #Pipet 100 µL from each tube onto the appropriate plate, and spread the mixture evenly across the plate. Incubate at 37°C overnight or approximately 16 hours. Position the plates | + | #Add 950 µL of SOC media per tube, and incubate at 37°C for 1 hours shaking at 200-300rpm. |
| + | #*Prepare the agar plates during this time: label them, and add sterile glass beads if using beads to spread the mixture. | ||
| + | #Pipet 100 µL from each tube onto the appropriate plate, and spread the mixture evenly across the plate. Incubate at 37°C overnight or approximately 16 hours. Position the plates with the agar side at the top, and the lid at the bottom. | ||
#Count the number of colonies on a light field or a dark background, such as a lab bench. Use the following equation to calculate your competent cell efficiency. If you've done triplicates of each sample, use the average cell colony count in the calculation. | #Count the number of colonies on a light field or a dark background, such as a lab bench. Use the following equation to calculate your competent cell efficiency. If you've done triplicates of each sample, use the average cell colony count in the calculation. | ||
#*(colonies on plate) / ng of DNA plated x 1000ng/µg | #*(colonies on plate) / ng of DNA plated x 1000ng/µg | ||
| Line 57: | Line 64: | ||
Competent cells should have an efficiency of 1.5x10^8 to 6x10^8 cfu/µg DNA, where "cfu" means "colony-forming unit" and is a measurement of cells. | Competent cells should have an efficiency of 1.5x10^8 to 6x10^8 cfu/µg DNA, where "cfu" means "colony-forming unit" and is a measurement of cells. | ||
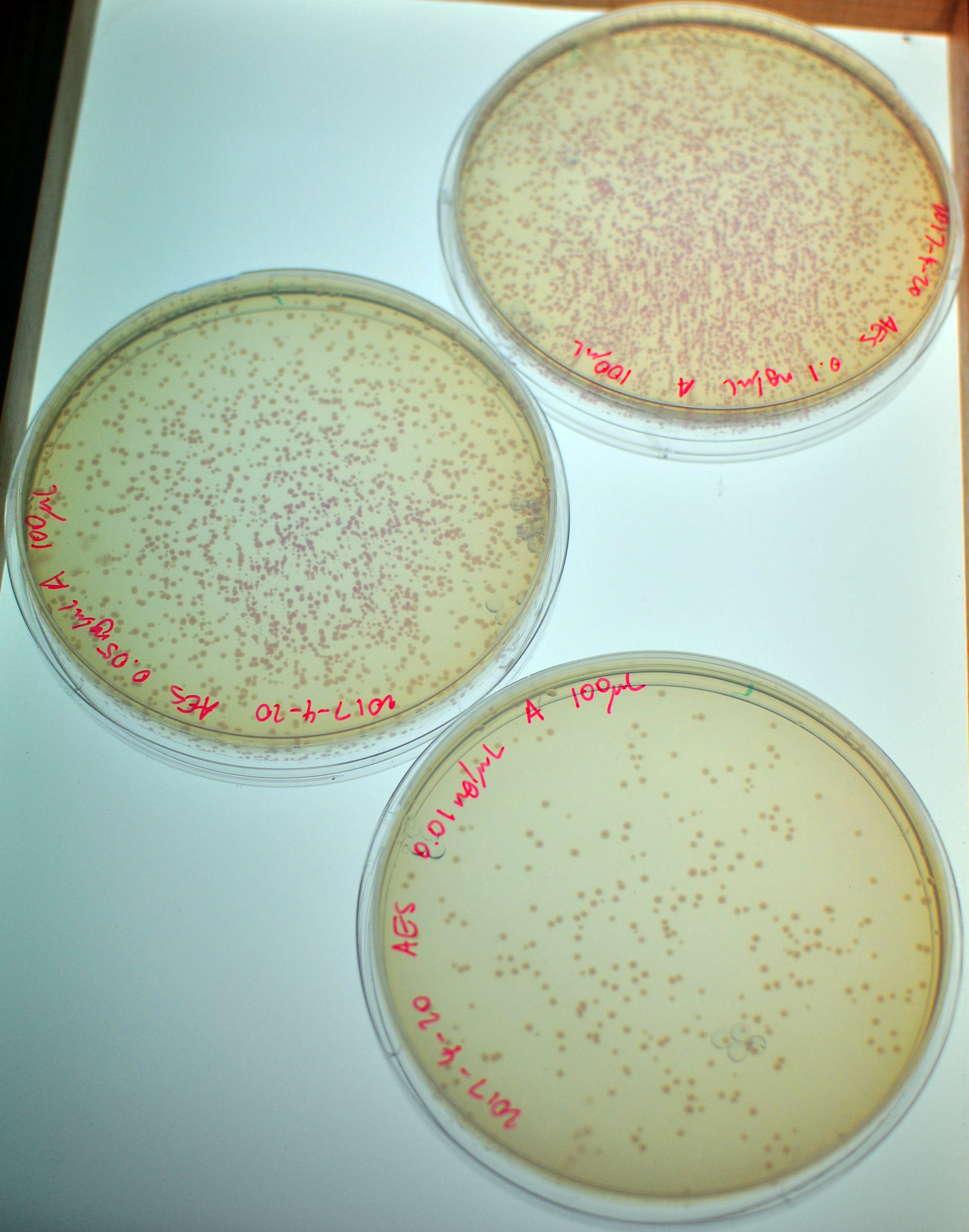
| − | [[File:2017 CCTK Results.jpeg| | + | [[File:2017 CCTK Results.jpeg|300px|thumb|right|Sample results for transformations using the Competent Cell Test Kit]] |
Here are some sample results: | Here are some sample results: | ||
Revision as of 16:03, 24 April 2017
- Registry Help Pages:
- TOC
- At-a-Glance
- FAQ
| Competent Cell Test Kit |

|
|
Before using your competent cells in an experiment, use the Competent Cell Test Kit to test the efficiency of your competent cells! The kit includes three vials of purified plasmid DNA from BBa_J04450 (RFP construct) in plasmid backbone pSB1C3. Each vial contains 50 µL of DNA at a different concentration: 100 pg/µL, 50 pg/µL, 10 pg/µL. Perform transformations with each of these to determine how efficient your competent cells are. It is important to have efficient competent cells because transformations performed with ligation products usually do not yield as many colonies due to the low DNA concentration in the ligation mixture. This means that you may see different results doing this test than you will at the end of the 3A Assembly protocol, or any other ligation. You can use the following suggested protocol for heat-shock transformation to test your chemically-competent E. coli cells. |
Materials needed
- 70% ethanol
- Paper towels
- Lab marker / Sharpie
- 1.5 mL microcentrifuge tubes
- Container for ice
- Ice
- Competent cell aliquot(s)
- Competent Cell Test Kit
- Agar plates with chloramphenicol
- 42°C Waterbath (or hot water source and thermometer)
- 37°C Incubators (oven and shaker)
- SOC media
- Sterile glass beads or sterile cell spreader
- Pipettor
- Pipette tips
Protocol
estimated time: 30 minutes active, 1.5 hours incubation
- Clean your working area by wiping down with 70% ethanol.
- Thaw competent cells on ice. Label one 1.5 mL microcentrifuge tubes for each transformation and then pre-chill by placing the tubes on ice.
- Do triplicates (3 each) of each concentration if possible, so you can calculate an average colony yield.
- Spin down the DNA tubes from the Competent Cell Test Kit/Transformation Efficiency Kit to collect all of the DNA into the bottom of each tube prior to use. A quick spin of 20-30 seconds at 8,000-10,000 rpm will be sufficient. Note: There should be 50 µL of DNA in each tube sent in the Kit.
- Pipet 1 µL of DNA into each microcentrifuge tube.
- Pipet 50 µL of competent cells into each tube. Flick the tube gently with your finger to mix.
- Incubate on ice for 30 minutes.
- Pre-heat waterbath now to 42°C. Otherwise, hot water and an accurate thermometer works, too!
- Heat-shock the cells by placing into the waterbath for 45 seconds (no longer than 1 min). Be careful to keep the lids of the tubes above the water level, and keep the ice close by.
- Immediately transfer the tubes back to ice, and incubate on ice for 5 minutes.
- Add 950 µL of SOC media per tube, and incubate at 37°C for 1 hours shaking at 200-300rpm.
- Prepare the agar plates during this time: label them, and add sterile glass beads if using beads to spread the mixture.
- Pipet 100 µL from each tube onto the appropriate plate, and spread the mixture evenly across the plate. Incubate at 37°C overnight or approximately 16 hours. Position the plates with the agar side at the top, and the lid at the bottom.
- Count the number of colonies on a light field or a dark background, such as a lab bench. Use the following equation to calculate your competent cell efficiency. If you've done triplicates of each sample, use the average cell colony count in the calculation.
- (colonies on plate) / ng of DNA plated x 1000ng/µg
- Note: The measurement "ng of DNA plated" refers to how much DNA was plated onto each agar plate, not the total amount of DNA used per transformation. You can calculate this number using the following equation:
- 1 µL x concentration of DNA (refer to vial) x (volume plated / total reaction volume)
Results
Competent cells should have an efficiency of 1.5x10^8 to 6x10^8 cfu/µg DNA, where "cfu" means "colony-forming unit" and is a measurement of cells.
Here are some sample results:
| DNA concentration | 10 pg/µL | 50 pg/µL | 100 pg/µL |
| # of colonies | 280 - 360 | 500 - 1000+ | 1000+ |
You can download Transformation Efficiency Calculation.xls for convenient calculation of the efficiencies. (mosthege 03:07, 22 May 2014 (CDT))