Difference between revisions of "Part:BBa K1937002:Design"
| Line 12: | Line 12: | ||
<b>Creation:</b> | <b>Creation:</b> | ||
| − | The BBa_K1937002 part contains the repU origin of replication of <i>B. subtilis</i> and the kanamycin resistance gene for <i>B. subtilis</i>. | + | The BBa_K1937002 part contains the repU origin of replication of <i>B. subtilis</i> and the kanamycin resistance gene for <i>B. subtilis</i>. <br> |
| − | + | ||
[[file:BBa_K1937002-map.jpg]] | [[file:BBa_K1937002-map.jpg]] | ||
| − | + | <b style="font-size:10px;"> <center><br>Figure 1: scheme of the Orikan part with <i>Bacillus subtilis</i> origin <i>repU</i> and a kanamycine resistance cassette.</center></b> | |
| − | + | <br>It was obtained by amplifying the repU-Kan region of pSB<sub>Bs</sub>0K-P (BBa_K1351040 or BBa_K823026) using primers OriKan forward (5’ cacagaatcaggggataacgcaggaaagaaACATGTAGTTATAAGTGACTAAACAAATAACTAAATAGATGGG) and OriKan reverse (5’ gttcctggccttttgctggccttttgctcaACATGTCGCAAAATGGCCCGATTTAAG). The resulting fragment was sub-cloned in the pSB<sub>1</sub>C<sub>3</sub> between the EcoRI and PstI restriction sites. | |
| + | <br> | ||
| + | [[file:Toulouse_France_backbone2.jpg]] | ||
| + | <b style="font-size:10px;"> <center><br>Figure 2: creation of the OriKan cassette. Position of the primers are indicated by the blue arrow on pSBBS0K-P (left part of the figure). The resulting PCR fragment is the blue pointed line. After digestion by EcoRI and PstI and ligation in the pSB1C3 plasmid, the resulting pSB1C3-OriKan plasmid was obtained (right part of the figure).</center></b> | ||
| + | <br> | ||
<b>Validation:</b> | <b>Validation:</b> | ||
| − | We successfully transformed <i>E. coli</i> and selected clones on chloramphenicol. Likewise, we successfully transformed <i>B. subtilis</i> and selected clones on kanamycine. Presence of the pSB<sub>1</sub>C<sub>3</sub>-OriKan plasmid was demonstrated in <i>B. subtilis</i> by PCR checking. The part sequence has been verified by sequencing. | + | We successfully transformed <i>E. coli</i> and selected clones on chloramphenicol. Likewise, we successfully transformed <i>B. subtilis</i> and selected clones on kanamycine. Presence of the pSB<sub>1</sub>C<sub>3</sub>-OriKan plasmid was demonstrated in <i>B. subtilis</i> by PCR checking. |
| + | <br> | ||
| + | [[file:Toulouse_France_backbone3.jpg]] | ||
| + | <b style="font-size:10px;"> <center><br>Figure 3: validation of the pSB1C3-Orikan presence in B. subtilis. PCR on colonies was performed using primers hybridizing in the kanamycine resistance gene and in the suffix. The colonies were issued from the transformation of B. subtilis by pSB1C3-Orikan (assays), by the pSBBS0K-P plasmid (negative control), or from the transformation of E. coli by pSB1C3-Orikan (positive control).</center></b> | ||
| + | <br>The part sequence has been verified by sequencing. | ||
More information available at http://2016.igem.org/Team:Toulouse_France/Description | More information available at http://2016.igem.org/Team:Toulouse_France/Description | ||
Revision as of 19:15, 29 October 2016
Part: BBa_K1937002 (pSB1C3-OriKan)
(Chassis E. coli/B. subtilis, carrier plasmid pSB1C3) Length: 2510 bp
Background: While Bacillus subtilis is of huge interest for a growing number of iGEM projects, it is not easy to develop new parts as it is required that they registered after sub-cloning in the E. coli plasmid pSB1C3. During the iGEM Toulouse 2016 project, we had the idea to create a part that could turn any pSB1C3-based plasmid in a shuttle vector (E. coli/B. subtilis). This BioBrick is a part developed by the Toulouse 2016 iGEM team (http://2016.igem.org/Team:Toulouse_France).
More information available at http://2016.igem.org/Team:Toulouse_France/Description
Creation:
The BBa_K1937002 part contains the repU origin of replication of B. subtilis and the kanamycin resistance gene for B. subtilis.

Figure 1: scheme of the Orikan part with Bacillus subtilis origin repU and a kanamycine resistance cassette.
It was obtained by amplifying the repU-Kan region of pSBBs0K-P (BBa_K1351040 or BBa_K823026) using primers OriKan forward (5’ cacagaatcaggggataacgcaggaaagaaACATGTAGTTATAAGTGACTAAACAAATAACTAAATAGATGGG) and OriKan reverse (5’ gttcctggccttttgctggccttttgctcaACATGTCGCAAAATGGCCCGATTTAAG). The resulting fragment was sub-cloned in the pSB1C3 between the EcoRI and PstI restriction sites.
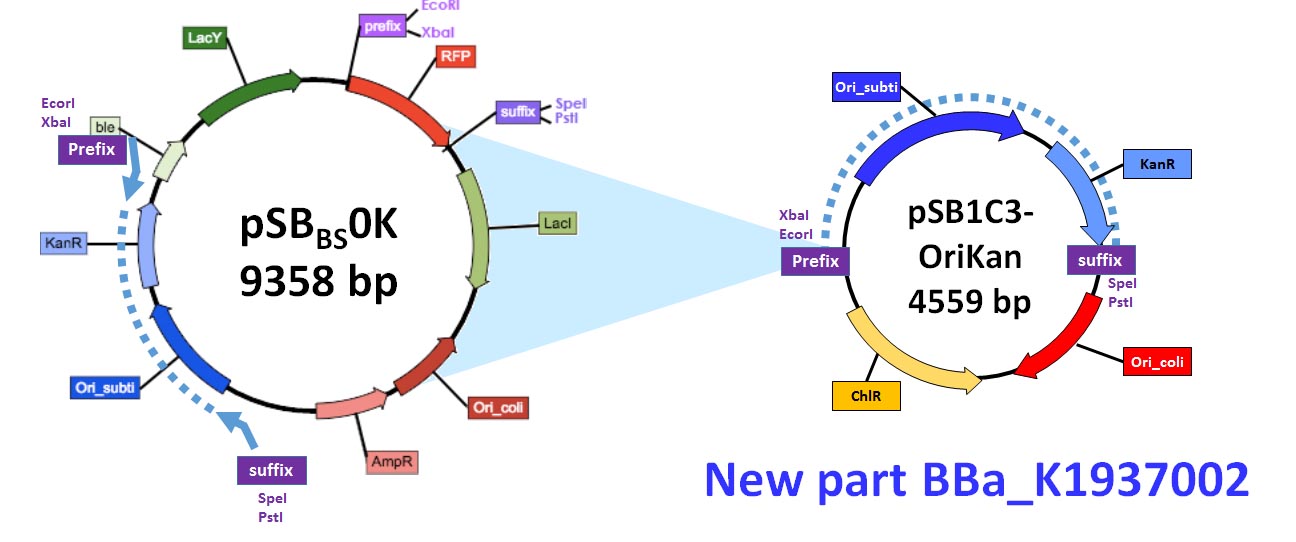
Figure 2: creation of the OriKan cassette. Position of the primers are indicated by the blue arrow on pSBBS0K-P (left part of the figure). The resulting PCR fragment is the blue pointed line. After digestion by EcoRI and PstI and ligation in the pSB1C3 plasmid, the resulting pSB1C3-OriKan plasmid was obtained (right part of the figure).
Validation:
We successfully transformed E. coli and selected clones on chloramphenicol. Likewise, we successfully transformed B. subtilis and selected clones on kanamycine. Presence of the pSB1C3-OriKan plasmid was demonstrated in B. subtilis by PCR checking.

Figure 3: validation of the pSB1C3-Orikan presence in B. subtilis. PCR on colonies was performed using primers hybridizing in the kanamycine resistance gene and in the suffix. The colonies were issued from the transformation of B. subtilis by pSB1C3-Orikan (assays), by the pSBBS0K-P plasmid (negative control), or from the transformation of E. coli by pSB1C3-Orikan (positive control).
The part sequence has been verified by sequencing.
More information available at http://2016.igem.org/Team:Toulouse_France/Description
Interest:
This part can be sub-cloned in any pSB1C3 plasmid to make it compatible with B. subtilis. It will therefore greatly simplified the use of Bacillus subtilis as a chassis and the registration of new Bacillus-aimed parts.
Sequence:
Annotation:
repU : 431-1435
Kan : 1595-2365
