Difference between revisions of "Part:BBa K2127002"
| Line 6: | Line 6: | ||
BBa_K2127002 is a composite part which contains the coding region of the psbB with an added his-tag and a double stop codon. The composite site also contains a lac promotor, RBS,and terminator which allow for direct expression of this insert. Assembly was done via sirect synthesis from IDT technologies as a gBlock. As a result no scar sites exist within the composite part. The BioBrick is able to be used directly with E. coli for protein expression and can also be transitioned into cyanobacterium with some genetic work for expression of His-tagged photosystem II complexes. | BBa_K2127002 is a composite part which contains the coding region of the psbB with an added his-tag and a double stop codon. The composite site also contains a lac promotor, RBS,and terminator which allow for direct expression of this insert. Assembly was done via sirect synthesis from IDT technologies as a gBlock. As a result no scar sites exist within the composite part. The BioBrick is able to be used directly with E. coli for protein expression and can also be transitioned into cyanobacterium with some genetic work for expression of His-tagged photosystem II complexes. | ||
| + | |||
| + | ===Verification=== | ||
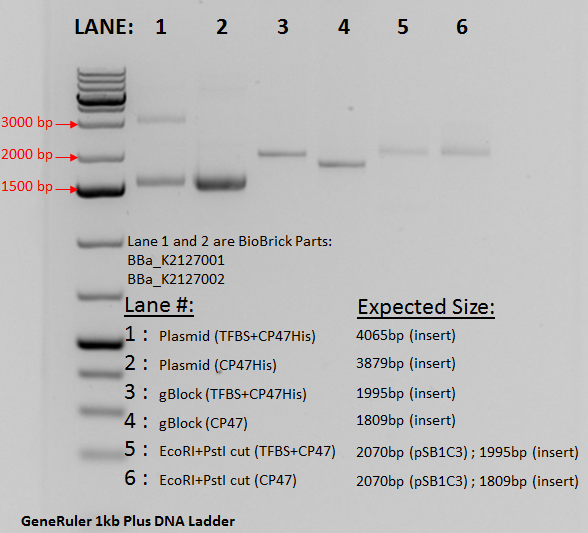
A plasmid containing the composite part was successfully made and transformed into E. coli DH5aF'Iq. The plasmid was extracted using miniprep and double digested using EcoRI and Psti. The gel below confirms the transformation. Lane 2 contains the undigested plasmid running at approximately 1500 bp due to a supercoiled nature. Lane 4 contains the gBlock from IDT and acts as a size control. The band is seen aa expected at 1809 bp. In lane 6, the digested plasmid is seen. Due to both the pSB1C3 vector and the psbB insert being very close to each other at 1809bp and 2070 bp interference did not allow for a clear separation of the bands. However compared to Lane 2 it can bee seen that digestion did occur since the run time changes significantly between the digested linear sample and the undigested supercoiled plasmid. | A plasmid containing the composite part was successfully made and transformed into E. coli DH5aF'Iq. The plasmid was extracted using miniprep and double digested using EcoRI and Psti. The gel below confirms the transformation. Lane 2 contains the undigested plasmid running at approximately 1500 bp due to a supercoiled nature. Lane 4 contains the gBlock from IDT and acts as a size control. The band is seen aa expected at 1809 bp. In lane 6, the digested plasmid is seen. Due to both the pSB1C3 vector and the psbB insert being very close to each other at 1809bp and 2070 bp interference did not allow for a clear separation of the bands. However compared to Lane 2 it can bee seen that digestion did occur since the run time changes significantly between the digested linear sample and the undigested supercoiled plasmid. | ||
| Line 16: | Line 18: | ||
Also in conjunction to its functions, CP47 also forms part of the quinone channel which allows direct transfer of electrons from the D1 monomer to quinones of the electron transport chain. | Also in conjunction to its functions, CP47 also forms part of the quinone channel which allows direct transfer of electrons from the D1 monomer to quinones of the electron transport chain. | ||
| + | |||
| + | ==Functionality== | ||
| + | |||
| + | [File:Team_Ingenuity_Lab_CP47expression|thumb|500px|centre|]] | ||
Revision as of 23:44, 27 October 2016
CP47 His-tagged with Lac Promoter
The psbB gene codes for the CP-47 subunit of the Cyanobacterial Photosystem II in Synechocystis sp. PCC 6803. Using site-directed mutagenesis Dr Frankel’s team developed a Synechocystis sp. PCC 6803 mutant containing a histidine tag at the C-Terminus of CP47 subunit. Using the His-Tag, the Photosystem II complex can be isolated and analyzed the activity using an electron evolution analyzer.
BBa_K2127002 is a composite part which contains the coding region of the psbB with an added his-tag and a double stop codon. The composite site also contains a lac promotor, RBS,and terminator which allow for direct expression of this insert. Assembly was done via sirect synthesis from IDT technologies as a gBlock. As a result no scar sites exist within the composite part. The BioBrick is able to be used directly with E. coli for protein expression and can also be transitioned into cyanobacterium with some genetic work for expression of His-tagged photosystem II complexes.
Verification
A plasmid containing the composite part was successfully made and transformed into E. coli DH5aF'Iq. The plasmid was extracted using miniprep and double digested using EcoRI and Psti. The gel below confirms the transformation. Lane 2 contains the undigested plasmid running at approximately 1500 bp due to a supercoiled nature. Lane 4 contains the gBlock from IDT and acts as a size control. The band is seen aa expected at 1809 bp. In lane 6, the digested plasmid is seen. Due to both the pSB1C3 vector and the psbB insert being very close to each other at 1809bp and 2070 bp interference did not allow for a clear separation of the bands. However compared to Lane 2 it can bee seen that digestion did occur since the run time changes significantly between the digested linear sample and the undigested supercoiled plasmid.
Usage and Biology
The his-tagged CP47 subunit is approximately 48kDa and is a transmembrane protein. As part of the Photosystem II complex, the subunit binds chlorophyll and acts as part of the light capturing array. The subunit ligand sites allow for electron movement across the peptide to facilitate movement within the electron transfer chain from both the light capturing array and from the photolysis of water. The subunit also composes part of the manganese calcium complex for oxygen evolution. It is extremely integral to the function of the PSII complex.
Also in conjunction to its functions, CP47 also forms part of the quinone channel which allows direct transfer of electrons from the D1 monomer to quinones of the electron transport chain.
Functionality
[File:Team_Ingenuity_Lab_CP47expression|thumb|500px|centre|]]
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 473
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 597
Illegal AgeI site found at 1194
Illegal AgeI site found at 1398 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 918
Illegal SapI site found at 1595