Difference between revisions of "Part:BBa K1933001"
(→Usage and Biology) |
|||
| Line 3: | Line 3: | ||
<partinfo>BBa_K1933001 short</partinfo> | <partinfo>BBa_K1933001 short</partinfo> | ||
| − | INPNC codes for N- and C- terminal domain of Ice-Nucleation Protein (INP) from '' Pseudomonas syringae '', | + | INPNC codes for N- and C- terminal domain of Ice-Nucleation Protein (INP) from '' Pseudomonas syringae '', fused to a 6xHis tag to be easily identified by Western blotting[1]. This is used as an anchoring motif for the cell surface display of passengers. This domain does not have signal peptide for anchoring on the cell surface[2]. Surface displayed passengers retain enzyme activity even when it is linked to INPNC with 6xHis tag. |
<br>For more information, please visit [http://2016.igem.org/Team:Kyoto our wiki] | <br>For more information, please visit [http://2016.igem.org/Team:Kyoto our wiki] | ||
| Line 12: | Line 12: | ||
==Usage and Biology== | ==Usage and Biology== | ||
| − | Ice | + | Ice Nucleation Protein(INP) used for our surface display systems is produced by P. syringae, a famous model organism among ice nucleation active (INA) bacteria. |
| − | Although the freezing point of water is 0°C, pure water can supercool to -40°C before homogeneous nucleation randomly | + | Although the freezing point of water is 0°C, pure water can supercool to -40°C before homogeneous ice nucleation randomly occur to form ice crystals. Generally, minimally impure water will freeze spontaneously before -15°C. |
| − | Generally, minimally impure water will freeze spontaneously before -15°C. | + | However, INP produced by P. syringae causes ice nucleation as high as -2°C. This bacteria use this property to form frosts on plants, causing damage and making the plants release nutrients for their growth |
| − | However, INP produced by | + | |
| − | + | ||
<br>INP are expressed as the ice nucleation site, expressed in the outer cell membrane. Modified INP are used as a surface expression system. | <br>INP are expressed as the ice nucleation site, expressed in the outer cell membrane. Modified INP are used as a surface expression system. | ||
| Line 28: | Line 26: | ||
We ''' added His-tag ''' to <big>[https://parts.igem.org/Part:BBa_K811005 BBa_K811005]</big>. This addition introduces two new functions to the fusion protein: | We ''' added His-tag ''' to <big>[https://parts.igem.org/Part:BBa_K811005 BBa_K811005]</big>. This addition introduces two new functions to the fusion protein: | ||
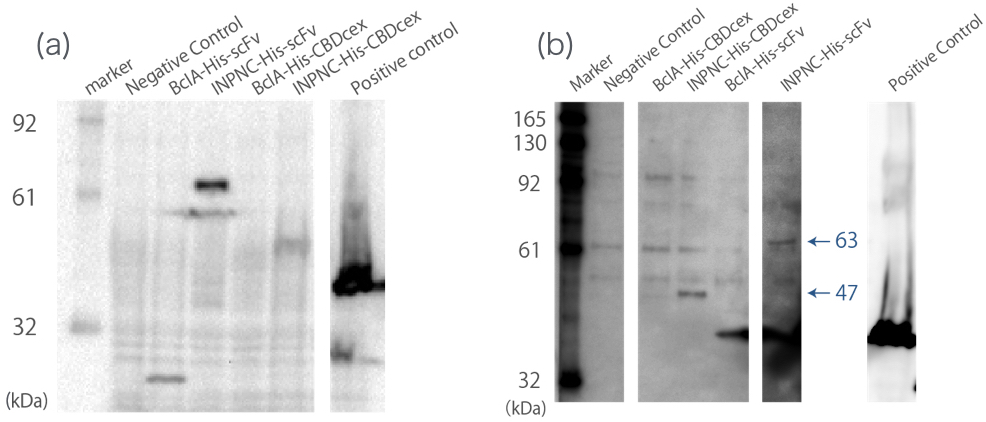
<br><br>First, we can use anti-His tag antibody to detect their surface expression regardless of passenger protein placed downstream. We confirmed this with detections of the INPNC-His-(passenger protein) by Western blotting using anti-His tag antibody. (Fig.1(a)) | <br><br>First, we can use anti-His tag antibody to detect their surface expression regardless of passenger protein placed downstream. We confirmed this with detections of the INPNC-His-(passenger protein) by Western blotting using anti-His tag antibody. (Fig.1(a)) | ||
| − | <br><br>Second, INPNC-His-(passenger protein) can be purified using His tag’s affinity to nickel columns even in denaturing conditions to recollect it from membrane fractions of E.coli. This would allow fusion protein detection even if the protein’s expression levels are low, further strengthening the first function. We tested this function with Western | + | <br><br>Second, INPNC-His-(passenger protein) can be purified using His tag’s affinity to nickel columns even in denaturing conditions to recollect it from membrane fractions of E.coli. This would allow fusion protein detection even if the protein’s expression levels are low, further strengthening the first function. We tested this function with Western blotting. Sample was prepared by obtaining the membrane fraction from the E. coli lysate by ultracentrifugation, and solubilized them using 7M guanidine-HCl, then purification of the target protein using Nickel Sepharose. (Fig.1(b)) |
| − | blotting. Sample was prepared by obtaining the membrane fraction from the E. coli lysate by ultracentrifugation, and solubilized them using 7M guanidine-HCl, then purification of the target protein using Nickel Sepharose. (Fig.1(b)) | + | |
Revision as of 06:19, 18 October 2016
INPNC fused to 6xHis tag
INPNC codes for N- and C- terminal domain of Ice-Nucleation Protein (INP) from Pseudomonas syringae , fused to a 6xHis tag to be easily identified by Western blotting[1]. This is used as an anchoring motif for the cell surface display of passengers. This domain does not have signal peptide for anchoring on the cell surface[2]. Surface displayed passengers retain enzyme activity even when it is linked to INPNC with 6xHis tag.
For more information, please visit [http://2016.igem.org/Team:Kyoto our wiki]
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 952
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 72
Illegal NgoMIV site found at 405
Illegal AgeI site found at 823 - 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Ice Nucleation Protein(INP) used for our surface display systems is produced by P. syringae, a famous model organism among ice nucleation active (INA) bacteria.
Although the freezing point of water is 0°C, pure water can supercool to -40°C before homogeneous ice nucleation randomly occur to form ice crystals. Generally, minimally impure water will freeze spontaneously before -15°C.
However, INP produced by P. syringae causes ice nucleation as high as -2°C. This bacteria use this property to form frosts on plants, causing damage and making the plants release nutrients for their growth
INP are expressed as the ice nucleation site, expressed in the outer cell membrane. Modified INP are used as a surface expression system.
We omitted stop codon from this part to make BBa_K1933011.
We used BBa_K1933011 for construction of ☆BBa_K1933100, BBa_K1933101, ☆BBa_K1933200.
Characterization
We improved BBa_K811005 from Penn 2012!!
Western blotting
We added His-tag to BBa_K811005. This addition introduces two new functions to the fusion protein:
First, we can use anti-His tag antibody to detect their surface expression regardless of passenger protein placed downstream. We confirmed this with detections of the INPNC-His-(passenger protein) by Western blotting using anti-His tag antibody. (Fig.1(a))
Second, INPNC-His-(passenger protein) can be purified using His tag’s affinity to nickel columns even in denaturing conditions to recollect it from membrane fractions of E.coli. This would allow fusion protein detection even if the protein’s expression levels are low, further strengthening the first function. We tested this function with Western blotting. Sample was prepared by obtaining the membrane fraction from the E. coli lysate by ultracentrifugation, and solubilized them using 7M guanidine-HCl, then purification of the target protein using Nickel Sepharose. (Fig.1(b))
These two results confirms the enhanced functions of INPNC parts (BBa_K811005) existing in the iGEM Regisrtry.
Please see ☆BBa_K1933100, BBa_K1933101, and BBa_K1933200 for full characterization of this part.
Judging
We fulfilled criteria listed below with this part.
- Validated Part/ Validated Contribution(Silver)
- Improve a previous part or project(Gold)
- [http://2016.igem.org/Team:Kyoto/Proof Proof of concept](Gold)
Reference
[1]Yim, Sung-Kun, et al. "Surface display of heme-and diflavin-containing cytochrome P450 BM3 in Escherichia coli: a whole cell biocatalyst for oxidation." Journal of microbiology and biotechnology, 20.4, (2010): 712-717.
[2]Park, Tae Jung, et al. "Surface display of recombinant proteins on Escherichia coli by BclA exosporium of Bacillus anthracis." Microbial cell factories, 12.1, (2013): 1.