Difference between revisions of "Part:BBa K1919300"
| Line 23: | Line 23: | ||
[[File:Figure1.png|450px|thumb|left|'''Figure 1:''' the physical position of six genes on genome of E.coli K12.]] | [[File:Figure1.png|450px|thumb|left|'''Figure 1:''' the physical position of six genes on genome of E.coli K12.]] | ||
| + | |||
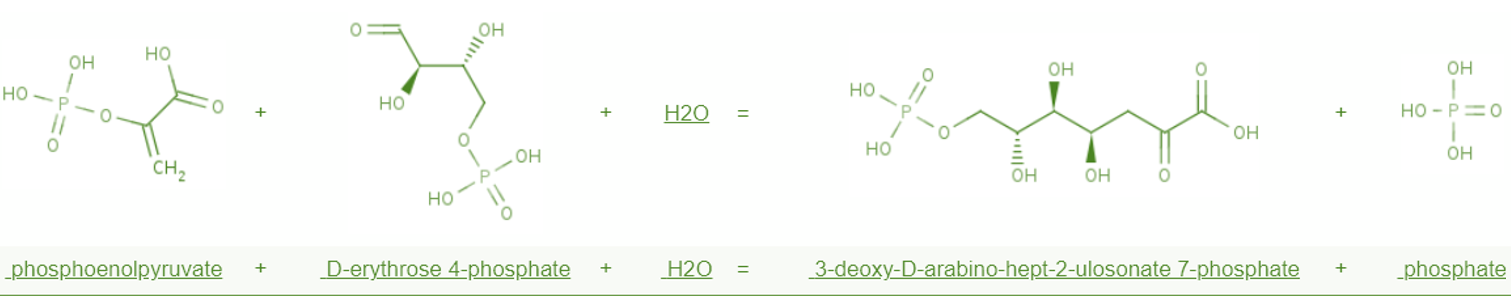
| + | This figure shows all six genes’ physical position in liv operon. Those six genes participate directly in branched chain amino acid transport. Two periplasmic amino acid binding protein are encoded by livJ and livK. These two proteins confer specificity on LIV-I and LS transport system. The livJ gene product binds leucine, isoleucine and valine, whereas the livK gene product is specific to leucine. These two system share a set of membrane components, products of the livHMGF genes. LivH and livM are estimated two transmembrane proteins locateing in innermenbrane of E.coli. An analysis of the livH and livM DNA sequence suggests that they encode hydrophobic proteins capable of spanning the membrane several times. The livG and livF proteins are less hydrophobic, but are also tightly associated with the membrane. Both livG and livF contain the consensus sequence for adenine nucleotide binding observed in many other transport proteins. | ||
Revision as of 02:33, 17 October 2016
livJ
LivJ encodes periplasmic binding protein for branched-chain amino acid ABC transporter which is encodes by operon livHMGF. The livJ gene product binds leucine, isoleucine, and valine. Please note that there is a short guide peptide on N terminal of livJ coding region, which is used to guide the precusor to be transported into periplasmic room. This device constructed in our team contains a promoter J23100, a RBS, livJ coding region and a terminator. The device can be transferred into E.coli and overexpress livJ directly. The expression of the livJ RNA in the part has been studied via qPCR.The quantified result suggests that compared with negtive control of E.coli (not cantain the plasmid), livJ has been highly expressed in E.coli BL21 and E.coli DH5a.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 382
Illegal AgeI site found at 203
Illegal AgeI site found at 485 - 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI site found at 366
Quantitative PCR exams expression levels of liv operon
Liv operon (leucine isoleucine valine) contains six genes. We cloned all those genes into overexpression vector in which livJKHMGF are under control of promoter J23100. All those devices have been transferred into E.coli and RNA expression levels are scrutinized via qPCR. In the trial, we regarded two genes, rrsA and dnaA, as reference. The rrsA gene transcripts 16s RNA while dnaA encodes a component of DNA polymerase. The expression level of rrsA is very high whereas the dnaA expression level is quite low. Generally speaking, the expression level of gene we need to study is always higher than dnaA and lower than rrsA. The two reference genes expression level are relatively stable.
This figure shows all six genes’ physical position in liv operon. Those six genes participate directly in branched chain amino acid transport. Two periplasmic amino acid binding protein are encoded by livJ and livK. These two proteins confer specificity on LIV-I and LS transport system. The livJ gene product binds leucine, isoleucine and valine, whereas the livK gene product is specific to leucine. These two system share a set of membrane components, products of the livHMGF genes. LivH and livM are estimated two transmembrane proteins locateing in innermenbrane of E.coli. An analysis of the livH and livM DNA sequence suggests that they encode hydrophobic proteins capable of spanning the membrane several times. The livG and livF proteins are less hydrophobic, but are also tightly associated with the membrane. Both livG and livF contain the consensus sequence for adenine nucleotide binding observed in many other transport proteins.