Difference between revisions of "Part:BBa K1694044"
| Line 3: | Line 3: | ||
<h1>'''Introduction:'''</h1> | <h1>'''Introduction:'''</h1> | ||
[[File:EGFRRFP.png|900px|thumb|center|'''Fig.1''' Pcons+B0034+Lpp-OmpA-N+scFv(anti-EGFR)+B0030+RFP+J61048]] | [[File:EGFRRFP.png|900px|thumb|center|'''Fig.1''' Pcons+B0034+Lpp-OmpA-N+scFv(anti-EGFR)+B0030+RFP+J61048]] | ||
| + | <p style="font-size:120%">'''Transformation of single plasmid'''</p> | ||
| − | + | To prove that our scFv can actually bind on to the antigens on carcinoma cells, we connected each scFv with a different fluorescence protein. Therefore, we can use fluorescent microscopes to clearly observe, if the ''E. coli'' has produced scFv proteins. Currently, we have built three different scFv connected with their respectively fluorescent protein, which are anti-VEGF+GFP, anti-EGFR+RFP, anti-HER2+BFP. The process of cell staining can identify the antigen distribution on cancer cells by observing the fluorescence. Furthermore, if we use the three scFv simultaneously, we can also detect multiple markers. | |
| − | To prove that our scFv can actually bind on to the | + | |
<br><br> | <br><br> | ||
<html> | <html> | ||
| − | Introduction | + | Introduction to basic parts: <br> |
<a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K1694002"> Lpp-OmpA-N</a><br> | <a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K1694002"> Lpp-OmpA-N</a><br> | ||
<a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K1694004"> Anti-EGFR </a><br> | <a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K1694004"> Anti-EGFR </a><br> | ||
| Line 17: | Line 17: | ||
<p style="font-size:120%">'''1.Cloning'''</p> | <p style="font-size:120%">'''1.Cloning'''</p> | ||
[[File:PROCRFPPCRsss.png|200px|thumb|left|'''Fig.2''' The PCR result of the Pcons+B0034+Lpp-OmpA-N+scFv(anti-EGFR)+B0030+GFP+J61048. The DNA sequence length is around 2000~2200 bp, so the PCR products should appear at 2200~2400 bp.]] | [[File:PROCRFPPCRsss.png|200px|thumb|left|'''Fig.2''' The PCR result of the Pcons+B0034+Lpp-OmpA-N+scFv(anti-EGFR)+B0030+GFP+J61048. The DNA sequence length is around 2000~2200 bp, so the PCR products should appear at 2200~2400 bp.]] | ||
| − | After assemble the DNA sequences from the basic parts, we recombined each Pcons+B0034+Lpp-OmpA-N+scFv(anti-EGFR)+B0030+RFP+J61048 gene to | + | After we assemble the DNA sequences from the basic parts, we recombined each Pcons+B0034+Lpp-OmpA-N+scFv(anti-EGFR)+B0030+RFP+J61048 gene to pSB1C3 backbones and conducted PCR experiments to ensure the size of each of the parts. The DNA sequence length of the these parts are around 2000~2200 bp. For PCR experiments, the size of products should be approximately 2200~2400 bp. The '''Fig.2''' below shows the correct size of this part, and proved that we successful ligated the sequence onto an ideal backbone. |
[[File:PROCRFP.png|600px|thumb|center|'''Fig.3''' Pcons+B0034+Lpp-OmpA-N+scFv(anti-EGFR)+B0030+RFP+J61048]] | [[File:PROCRFP.png|600px|thumb|center|'''Fig.3''' Pcons+B0034+Lpp-OmpA-N+scFv(anti-EGFR)+B0030+RFP+J61048]] | ||
| Line 27: | Line 27: | ||
''' (1) Cell staining experiment:''' | ''' (1) Cell staining experiment:''' | ||
| − | After | + | After inserting a part of the scFv gene into our ''E. coli'', we were going to prove that our detectors have successfully displayed a functional scFv of anti-EGFR. To prove this, we have decided to undergo the cell staining experiment by using our ''E. coli'' to detect the EGFR on the SKOV-3 cancer cell lines. SKOV-3 is a kind of epithelial cell that expressed markers such as EGFR. |
<br> | <br> | ||
<br> | <br> | ||
| Line 34: | Line 34: | ||
<br> | <br> | ||
<div style="display: block; height: 250pt;"> | <div style="display: block; height: 250pt;"> | ||
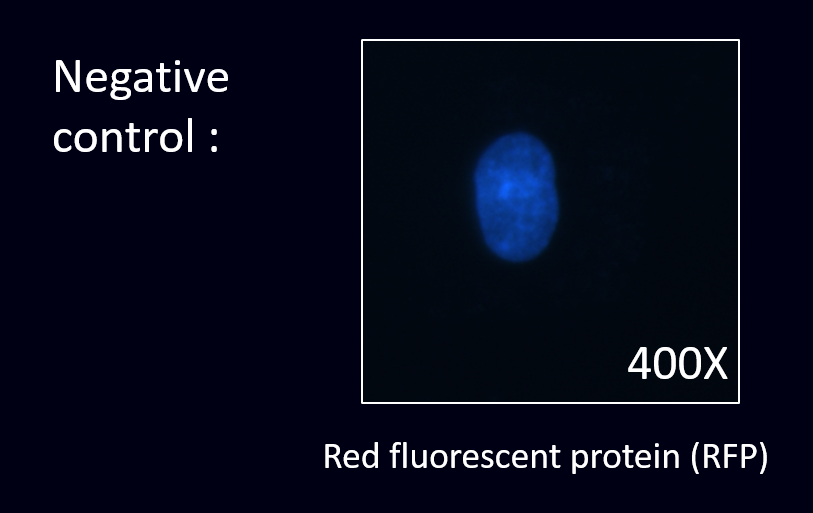
| − | [[File:Co3.png|400px|thumb|left|'''Fig.5''' As | + | [[File:Co3.png|400px|thumb|left|'''Fig.5''' As a result,there is no red fluorescent ''E. coli'' stick on the cell’s surface as there is no specific scFv displayed around the ''E. coli''.]] |
[[File:EGFPRFPCELL1.png|400px|thumb|left|'''Fig.6''' There are green fluorescent anti-EGFR ''E. coli'' stick on the cell’s surface as the anti-EGFR probes on ''E. coli'' successfully detect and bind with EGFR.]] | [[File:EGFPRFPCELL1.png|400px|thumb|left|'''Fig.6''' There are green fluorescent anti-EGFR ''E. coli'' stick on the cell’s surface as the anti-EGFR probes on ''E. coli'' successfully detect and bind with EGFR.]] | ||
</div> | </div> | ||
| Line 40: | Line 40: | ||
<h1>'''Modeling'''</h1> | <h1>'''Modeling'''</h1> | ||
| − | In the modeling part, we | + | In the modeling part, we discovered the optimum protein production time by using the genetic algorithm in Matlab. |
<br> | <br> | ||
We want to characterize the actual kinetics of this Hill-function based model that accurately reflects protein production time. | We want to characterize the actual kinetics of this Hill-function based model that accurately reflects protein production time. | ||
| Line 48: | Line 48: | ||
[[File:Anti-EGFR-RFP2.jpg|900px|thumb|center|'''Fig.7''' From this graph, the orange curve is the simulated protein expression. The blue curve is our experimental data. | [[File:Anti-EGFR-RFP2.jpg|900px|thumb|center|'''Fig.7''' From this graph, the orange curve is the simulated protein expression. The blue curve is our experimental data. | ||
| − | By comparing the orange curve and the blue curve, the blue curve | + | By comparing the orange curve and the blue curve, the blue curve has a close fit to the orange in the simulation. |
The orange curve reaches peak after growing about 17 hours. | The orange curve reaches peak after growing about 17 hours. | ||
| − | Thus, we can know that the E. Cotector can have maximum efficiency at this point.]] | + | Thus, we can know that the E.Cotector can have maximum efficiency at this point.]] |
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Revision as of 12:11, 22 September 2015
Pcons+B0034+Lpp-OmpA-N+scFv(Anti-EGFR)+B0030+RFP+J61048
Introduction:
Transformation of single plasmid
To prove that our scFv can actually bind on to the antigens on carcinoma cells, we connected each scFv with a different fluorescence protein. Therefore, we can use fluorescent microscopes to clearly observe, if the E. coli has produced scFv proteins. Currently, we have built three different scFv connected with their respectively fluorescent protein, which are anti-VEGF+GFP, anti-EGFR+RFP, anti-HER2+BFP. The process of cell staining can identify the antigen distribution on cancer cells by observing the fluorescence. Furthermore, if we use the three scFv simultaneously, we can also detect multiple markers.
Introduction to basic parts:
Lpp-OmpA-N
Anti-EGFR
Experiment
1.Cloning
After we assemble the DNA sequences from the basic parts, we recombined each Pcons+B0034+Lpp-OmpA-N+scFv(anti-EGFR)+B0030+RFP+J61048 gene to pSB1C3 backbones and conducted PCR experiments to ensure the size of each of the parts. The DNA sequence length of the these parts are around 2000~2200 bp. For PCR experiments, the size of products should be approximately 2200~2400 bp. The Fig.2 below shows the correct size of this part, and proved that we successful ligated the sequence onto an ideal backbone.
2. Transformation of single plasmid
(1) Cell staining experiment:
After inserting a part of the scFv gene into our E. coli, we were going to prove that our detectors have successfully displayed a functional scFv of anti-EGFR. To prove this, we have decided to undergo the cell staining experiment by using our E. coli to detect the EGFR on the SKOV-3 cancer cell lines. SKOV-3 is a kind of epithelial cell that expressed markers such as EGFR.
(2) Staining results:
Modeling
In the modeling part, we discovered the optimum protein production time by using the genetic algorithm in Matlab.
We want to characterize the actual kinetics of this Hill-function based model that accurately reflects protein production time.
When we have the simulated protein production rate, the graph of protein production versus time can be drawn. Thus, we get the optimum protein production time
Compared with the simulated protein production rate of time, our experiment data quite fit the simulation.

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 451
Illegal NgoMIV site found at 1987
Illegal AgeI site found at 1828
Illegal AgeI site found at 1940 - 1000COMPATIBLE WITH RFC[1000]