Difference between revisions of "Part:BBa K1694023"
CrystalWei (Talk | contribs) |
CrystalWei (Talk | contribs) |
||
| Line 24: | Line 24: | ||
<br> | <br> | ||
<br> | <br> | ||
| − | <b>Fig.9</b> -<b> Fig. 12 </b>are | + | <b>Fig.9</b> -<b> Fig. 12 </b>are cell staining results: |
<br> | <br> | ||
Negative control: | Negative control: | ||
Revision as of 05:45, 22 September 2015
Pcons+B0034+Lpp-OmpA-N+scFv(Anti-VEGF)
Introduction:
By ligating the constitutive promoter (BBa_J23101), strong ribosome binding site (BBa_B0034) and Lpp-OmpA-scFv, we were able to display scFv(Anti-VEGF) outside the E.coli cell membrane.
This year we want to provide a customized platform. We provide two libraries of Pcon+RBS+OmpA-scFv and Pcons+RBS+Fluorescence+Ter into E. coli. Therefore, our customers can choose any scfv and any fluorescence protein. Our team will then co-transform the two plasmids, which helps us tailor our product to the wishes of our customers.
Experiment
1.Cloning
After assemble the DNA sequences from the basic parts, we recombined each Pcons+RBS+Lpp-OmpA-N+scFv gene to PSB1C3 backbones and conducted a PCR experiment to check the size of each of the parts. The DNA sequence length of these parts is around 1100~1300 bp. In this PCR experiment, the scFv products size should be near at 1300~1500 bp. The Fig. 3 showed the correct size of the scFv, and proved that we successful ligated the scFv sequence onto an ideal backbone.
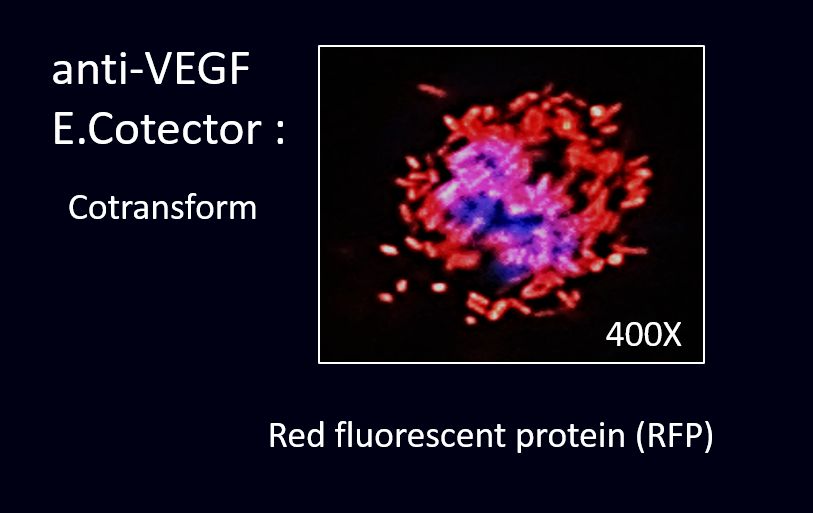
2.Cotransform
After cloning the part of anti-VEGF, we were able to co-transform anti-VEGF with different fluorescence protein into our E. coli.
The next step was to prove that our co-transformed product have successfully displayed scFv of anti-VEGF and expressed fluorescence protein.
To prove this, we conducted the cell staining experiment by using the co-transformed E. coli to detect VEGF in the cancer cell line.
Fig.9 - Fig. 12 are cell staining results:
Negative control:
There are red and green fluorescent anti-VEGF E. coli stick on the cell’s surfaces as the anti-VEGF probes on E. coli successfully detect and bind with VEGF.
Modeling
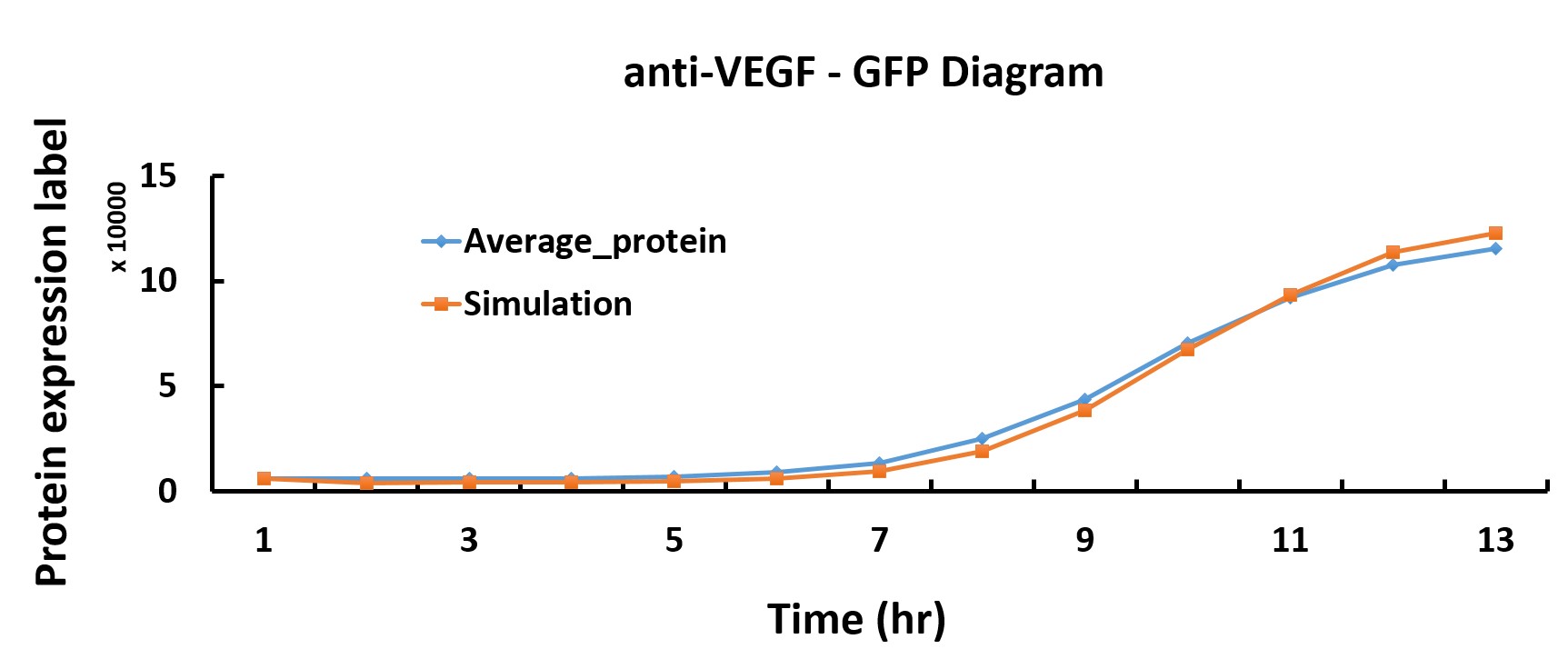
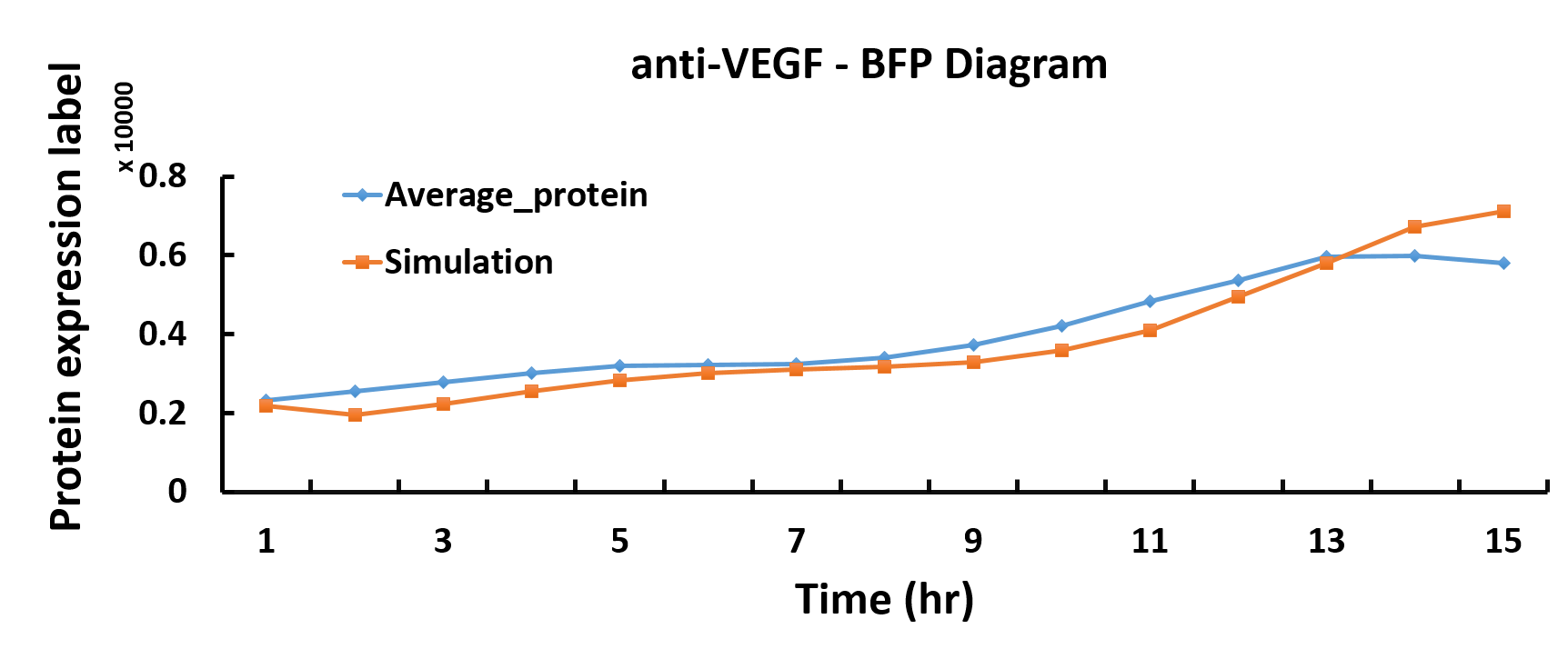
In the modeling part, we discover optimum protein production time by using the genetic algorithm in Matlab.
We want to characterize the actual kinetics of this Hill-function based model that accurately reflects protein production time.
When we have the simulated protein production rate, the graph of protein production versus time can be drawn. Thus, we get the optimum protein production time
Compared with the simulated protein production rate of time, our experiment data quite fit the simulation.
Co-transform

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 451
- 1000COMPATIBLE WITH RFC[1000]