Difference between revisions of "Part:BBa K1694004"
| Line 7: | Line 7: | ||
<p style="font-size:120%">'''ScFv (Single-Chain Variable Fragment)'''</p> | <p style="font-size:120%">'''ScFv (Single-Chain Variable Fragment)'''</p> | ||
| − | [[File:AE2.png|300px|thumb|right|<b>Fig.1 </b>Single-chain variable fragment and A coding gene of scFv (anti-EGFR)]] | + | [[File:AE2.png|300px|thumb|right|<b>Fig.1 </b>Single-chain variable fragment and A coding gene of scFv (anti-EGFR).]] |
ScFv (single-chain variable fragment) is a fusion protein containing light (VL) and heavy (VH) variable domains connected by a short peptide linker <b>(Fig. 1)</b>. The peptide linker (GGSSRSSSSGGGGSGGGG) is rich in glycine and serine which makes it flexible. | ScFv (single-chain variable fragment) is a fusion protein containing light (VL) and heavy (VH) variable domains connected by a short peptide linker <b>(Fig. 1)</b>. The peptide linker (GGSSRSSSSGGGGSGGGG) is rich in glycine and serine which makes it flexible. | ||
Revision as of 16:35, 20 September 2015
Single-chain variable fragment (Anti-EGFR)
Introduction:
ScFv (Single-Chain Variable Fragment)
ScFv (single-chain variable fragment) is a fusion protein containing light (VL) and heavy (VH) variable domains connected by a short peptide linker (Fig. 1). The peptide linker (GGSSRSSSSGGGGSGGGG) is rich in glycine and serine which makes it flexible.
Features of scFv:
1. Specific:Though remove of the constant regions , scFv still maintain the specificity of the original immunoglobulin.
2. Efficient:ScFv is smaller than the entire antibody, so that the loading of production to E.coli is lower.
Epidermal growth factor receptor
1. EGFR is a transmembrane tyrosine kinase receptor that regulates the cell division and cell apoptosis.
2. EGFR is characterized by an extracellular ligand-binding domain, a transmembrane domain, and a cytoplasmic domain containing the tyrosine kinase region followed by a carboxyl-terminal tail with tyrosine autophosphorylation sites.
3. EGFR is overexpressed on the cell surfaces of various solid tumors.
4. Mutations in the gene encoding EGFR that lead to overexpression of this protein will lead to uncontrollable cells proliferate.
Cetuximab
We selected the single chain variable fragments (scFv) of monoclonal antibodies Cetuximab and named it Anti-EGFR. Cetuximab (International Nonproprietary Name) is an epidermal growth factor receptor (EGFR) inhibitor. It is a chimeric (mouse/human) monoclonal antibody and it specific binds to target antigen epidermal growth factor receptor (EGFR). With high affinity it can prevent ligand binding and activation of signal transduction.
Mechanism:
1. Cetuximab inhibition
When Cetuximab binds to the extracellular domain of the EGFR, it prevents the activation and subsequent dimerization of the receptor, inhibition in signal transduction and anti-proliferative effects. Moreover, this agent may inhibit EGFR-dependent primary tumor growth and metastasis.
2. EGFR activation
Firstly, the ligand binding at the extracellular domain of EGFR will lead to the occurance of active homo- or hetero-dimers.
Dimerization induces the activation of the tyrosine kinase(TK) domain, leading to autophosphorylation of the receptors on multiple tyrosine residues.
This phosphorylation triggers recruitment of a range of adaptor proteins, , followed by a series of intracellular signaling cascades that finally will affect the cell proliferation, apoptosis, invasion, metastasis, and angiogenesis.
1.http://www.sciencedirect.com/science/article/pii/S0959804901002301
2.https://en.wikipedia.org/wiki/Cetuximab
3.http://www.ncbi.nlm.nih.gov/pubmed/22992668
Experiment:
After receiving the DNA sequences from the gene synthesis company, we recombined each scFv gene to pSB1C3 backbones and conducted a PCR experiment to check the size of each of the scFvs. The DNA sequence length of the scFvs are around 600~800 bp. In this PCR experiment, the scFv products size should be near at 850~1050 bp. The Fig.3 showed the correct size of the scFv (Anti-EGFR), and proved that we successful ligated the scFv sequence onto an ideal backbone.
Application of the part:
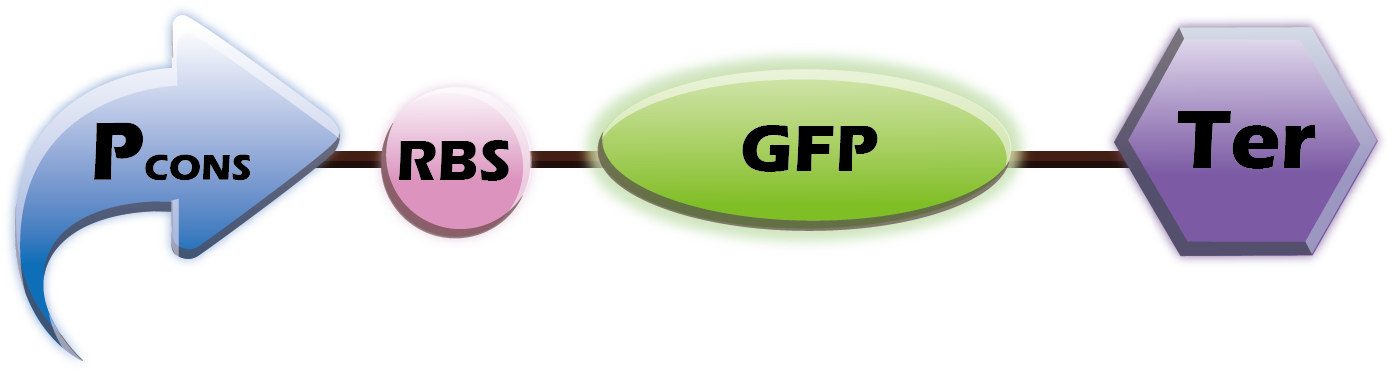
1. Co-transform (Two plasmids)
This year we want to provide a customized platform. We provide two libraries of Pcon+RBS+OmpA-scFv and Pcons+RBS+Fluorescence+Ter into E. coli. Therefore, our customers can choose any scfv and any fluorescence protein. Our team will then co-transform the two plasmids, which helps us tailor our product to the wishes of our customers.
(1) Parts:
(2) Cell staining experiment:
After cloning the part of anti-EGFR, we were able to co-transform anti-EGFR with different fluorescence protein into our E. coli.
The next step was to prove that our co-transformed product have successfully displayed scFv of anti-EGFR and expressed fluorescence protein.
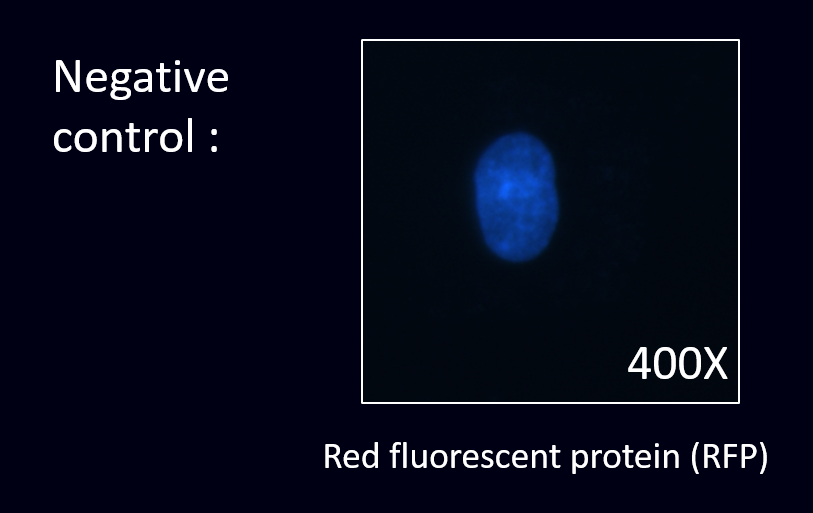
To prove this, we conducted the cell staining experiment by using the co-transformed E. coli to detect the EGFR in the cancer cell line.
(3) Staining results:
2. Transformation of single plasmid
To prove that our scFv can actually bind on to the antigen on cancer cells, we connected each scFv with a different fluorescence protein. Therefore we could use fluorescence microscope to clearly observe if the E. coli has produced scFv proteins. Currently, we built three different scFv connected with their respectively fluorescence protein. When applied on cell staining, we can identify the antigen distribution on cancer cells by observing the fluorescence. Furthermore, if we use the three scFv simultaneously, we can also detect multiple markers.
(1) Parts:
(2) Cell staining experiment:
After creating the part of scFv and transforming them into our E. coli, we were going to prove that our detectors have successfully displayed scFv of anti-EGFR. To prove this, we have decided to undergo the cell staining experiment by using our E. coli to detect the EGFR in the SKOV-3 cancer cell lines. SKOV-3 is a kind of epithelial cell that expressed markers such as EGFR.
(3) Staining results:
Modeling
In the modeling part, we discover optimum protein expression time by using the genetic algorithm(GA) in Matlab.
We want to characterize the actual kinetics of this Hill-function based model that accurately reflects protein expression time.
By using the differential function which was derived from these optimum parameters which were calculated by GA can help us to simulate the optimum protein expression.
When we have the simulated protein expression rate, the graph of protein expression versus time can be drawn.Thus, we can find the optimum protein expression time. However, the simulated protein expression curve is slower than the experimental curve by one hour. Therefore, to find the most exact optimum protein expression time, we infer that subtracting one hour of the optimum protein expression time would be correct.
1. Co-transform (Two plasmids)
This year we want to provide a customized platform. We provide two libraries of Pcon+RBS+OmpA-scFv and Pcons+RBS+Fluorescence+Ter into E. coli. Therefore, our customers can choose any scfv and any fluorescence protein. Our team will then co-transform the two plasmids, which helps us tailor our product to the wishes of our customers.



2. Transformation of single plasmid
To prove that our scFv can actually bind on to the antigen on cancer cells, we connected each scFv with a different fluorescence protein. Therefore we could use fluorescence microscope to clearly observe if the E. coli has produced scFv proteins. Currently, we built three different scFv connected with their respectively fluorescence protein. When applied on cell staining, we can identify the antigen distribution on cancer cells by observing the fluorescence. Furthermore, if we use the three scFv simultaneously, we can also detect multiple markers.



Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]