Difference between revisions of "Part:BBa K1635000"
(→Characterization) |
(→Characterization) |
||
| Line 25: | Line 25: | ||
| − | '''2015:Quantitative Analysis''' | + | '''2015: Quantitative Analysis''' |
The part BBa_K1635000 yielded a 142.23% of fluorescence compared to that of the BBa_K1383000 (Native) (figure 4). For all mutations considered, but not all developed into parts, calculated fluorescence ranged from that of the theoretical negative to 165.85% of the native (table 1). The "future parts" shown in table 1 are in the process of being developed into parts. This part falls into the higher range of fluorescence. We also created another point mutation at the same base as BBa_K1635000, but the fluorescence values recorded were similar (BBa_K1635000: 142.23%, and other mutation at same base: 137.96%). This part is a G to T point mutation, and the subsequent mutation is a G to A, and both of these give higher expression than G. | The part BBa_K1635000 yielded a 142.23% of fluorescence compared to that of the BBa_K1383000 (Native) (figure 4). For all mutations considered, but not all developed into parts, calculated fluorescence ranged from that of the theoretical negative to 165.85% of the native (table 1). The "future parts" shown in table 1 are in the process of being developed into parts. This part falls into the higher range of fluorescence. We also created another point mutation at the same base as BBa_K1635000, but the fluorescence values recorded were similar (BBa_K1635000: 142.23%, and other mutation at same base: 137.96%). This part is a G to T point mutation, and the subsequent mutation is a G to A, and both of these give higher expression than G. | ||
Revision as of 21:37, 19 September 2015
BBa_K1635000 (Archaeal translational efficiency part)
Usage and Biology
The ribosome binding sites (RBS) of archaea are not well characterized. By creating and characterizing a library of RBS sequences, researchers will be able to express proteins of interest at variable levels of expression in Methanococcus maripaludis. Ribosome binding sites are typically 6-7 base pair sequences on a transcript that is complementary to the 3’ end of the 16S rRNA. After binding of the RBS to the ribosome, translation will be initiated. An RBS with higher affinity for the ribosome will result in higher rate of translation, and inversely, an RBS with lower affinity will result in lower rate of translation.
Characterization
Characterization of the RBS sequences was accomplished using the red fluorescent protein, mCherry as a reporter. Qualitative analysis was evaluated by visualization of the RFP and quantitative analysis was completed through use of a plate reader for reading fluorescence.
2013: Improving the Construct and Characterization of Fluorescent Reporters for use in Methanogens
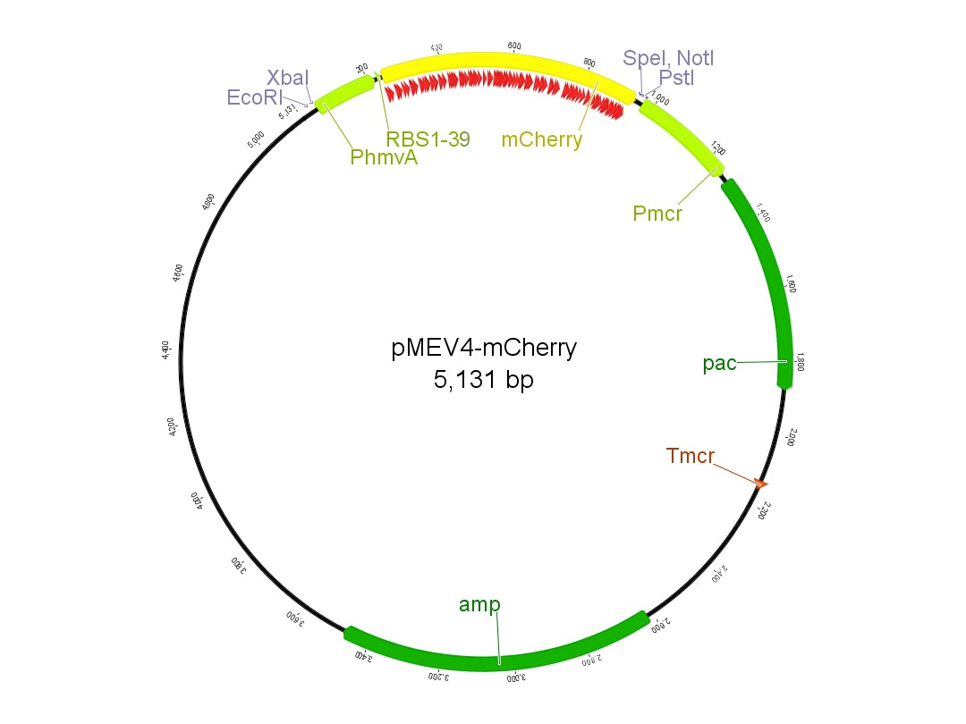
In 2013, the UGA-Georgia team submitted the part BBa_K1138002, named pAW50-mCherry. This construct, designed for use as a fluorescent reporter in methanogens, had a few issues. First, the part was not 100% biobrick compatible due to an internal restriction site in the mCherry gene. The characterization of the part was also inconsistent among samples, and overall unreproducible. To improve upon the previous part, we designed pMEV4-mCherry (BBa_K1383000, figure 1). The primary differences between this construct and the pAW50-mCherry of 2013 is that pMEV4-mCherry does not contain the internal restriction site and the selective resistance gene against puromycin, our antibiotic for use in M. maripaludis, has an independent promoter. Therefore, pMEV4-mCherry is 100% biobrick compatible, and can more reliably function under increased selective pressure. Improvements on characterization are elaborated more below.
2014: The Construct for Characterizing a Library of Ribosome Binding Site Sequences
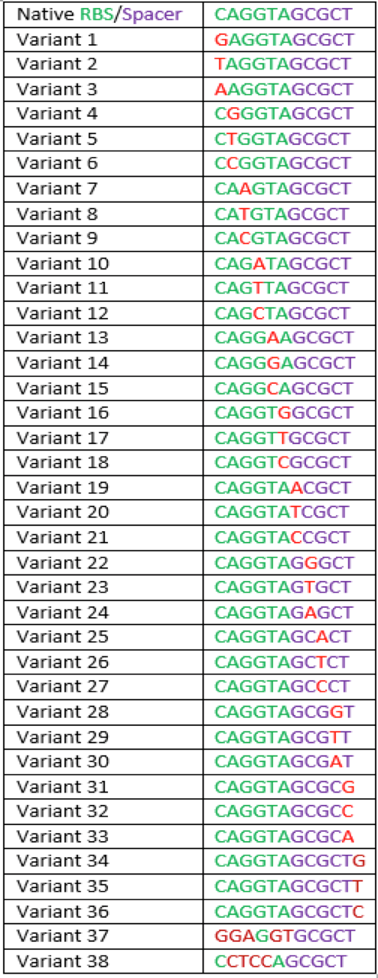
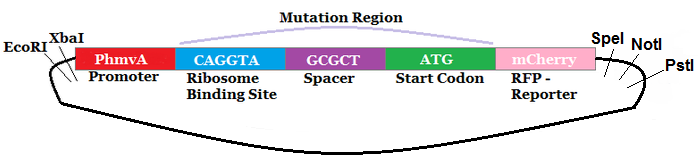
The region labeled 'RBS 1-39' in the pMEV4-mCherry vector (figure 1) is the site immediately upstream of the mCherry gene that will be subject to variation. Specifically, we will be mutating the 6 base pairs of the RBS, the 5 of the spacer, and the first base of the start codon. We begin with the 'native' RBS (BBa_K1383000, shown on this page), which is a known functional RBS sequence in methanogens and is typically used for creating synthetic parts. The RBS variants are created by making mutations on only one base at a time for every nucleotide different from the native (figure 2). We designed two additional RBS sequences based off the 16S rRNA data for M. maripaludis, termed theoretical 'perfect' and 'negative' (figure 2, #37 & #38, BBa_K1383001 & BBa_K1383002, respectively). These RBS sequences were designed to have the theoretical greatest and worst possible affinities for the 16S ribosome. Figure 3 illustrates the specific sequence for the part described on this page, the 'native' RBS.
2015: Quantitative Analysis
The part BBa_K1635000 yielded a 142.23% of fluorescence compared to that of the BBa_K1383000 (Native) (figure 4). For all mutations considered, but not all developed into parts, calculated fluorescence ranged from that of the theoretical negative to 165.85% of the native (table 1). The "future parts" shown in table 1 are in the process of being developed into parts. This part falls into the higher range of fluorescence. We also created another point mutation at the same base as BBa_K1635000, but the fluorescence values recorded were similar (BBa_K1635000: 142.23%, and other mutation at same base: 137.96%). This part is a G to T point mutation, and the subsequent mutation is a G to A, and both of these give higher expression than G.
More information on our protocols on developing the parts and quantifying their fluorescence values can be seen on UGA-Geogia 2015 Experiments page.
<" ">
">
Figure 4. Point mutations vs. fluorescence in terms of percentage against the native sequence (BBa_K1383000). The point mutation for this part is designated by the white circle. The sequence shown at "100%" is the native sequence (BBa_K1383000. All other represented mutations are in the process of being developed into parts in order to expand our expression library.
UGA-Georgia 2015 Table 1: This table shows the percent of native expression for 8 different mutations of the native sequence as well as the native sequence. This table includes both pre-existing parts and what will become future parts.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]