Difference between revisions of "Part:BBa K1761003"
(figure) |
(Bioluminiscence) |
||
| Line 50: | Line 50: | ||
''Figure 7: Fluorescence results of OmpX -mNeonGreen and OmpX - NanoLuc'' | ''Figure 7: Fluorescence results of OmpX -mNeonGreen and OmpX - NanoLuc'' | ||
| + | == Bioluminescence Confirmation == | ||
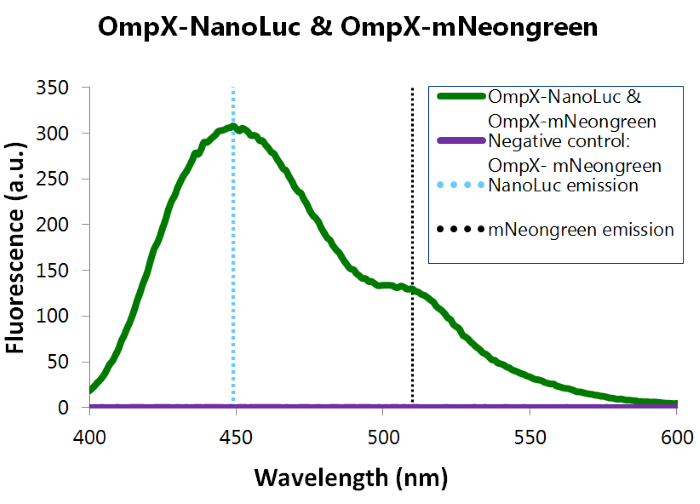
| + | To confirm whether mNeonGreen forms a good BRET (Bioluminescence resonance energy transfer) couple with NanoLuc, a bioluminescence measurement was performed (see Figure 8). In this experiment OmpX-NanoLuc's & OmpX-mNeonGreen's presence in the cells were tested by adding Nano-Glo substrate to the cells and measuring bioluminescence with the spectrophotometer. Figure 8 shows that OmpX-NanoLuc shows a peak characteristic for NanoLuc, indicating NanoLuc's presence within the cells. Moreover, the spectrogram shows a distinct shoulder near 517 nm, the emission wavelength of mNeongreen. Since no laser was used, excitation of mNeongreen can only be accomplished by NanoLuc so BRET (Bioluminescence resonance energy transfer) occured. This signal is measured when no click reaction is performed on the complex, meaning that this can be seen as the background noise of the sensor of iGEM team TU Eindhoven 2015. For more information about how to perform a bioluminescence measurement, see our Protocol Page [http://2015.igem.org/Team:TU_Eindhoven/Project/Protocols]. | ||
| + | |||
| + | [[File:TU_Eindhoven_results_bioluminiscence_neongreen_and_NanoLuc.png]] | ||
| + | |||
| + | ''Figure 8: Bioluminiscence results NanoLuc and mNeonGreen.'' | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Revision as of 20:09, 18 September 2015
mNeonGreen
mNeonGreen is a yellow-green fluorescent protein. For a fluorescene measurement, the mNeonGreen fluorophore can be excitated with a laser with a wavelenght of 480 nm and readed out at 517 nm.
Usage and Biology
mNeonGreen is a yellow-green fluorescent protein and is derived from a tetrameric fluorescent protein from cephalochordate Branchiostoma lanceolatum (see Figure 1). mNeonGreen is the brightest monomeric green of yellow fluorescent protein yet described and is an excellent fluorescence resonance energy transfer (FRET) acceptor for the newest cyan fluorescent proteins. [1]
Figure 1: Schamatic 3D structure of mNeonGreen
Gene Design
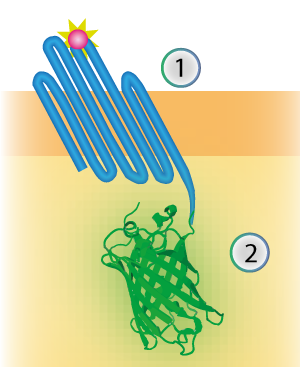
mNeonGreen was characterized by Shaner et al [1]. We inserted mNeonGreen in the pETDuet-1 vector together with OmpX and a BamHI-linker (see Figure 2) [1]. This linker is a 213 bp long flexible GGSGGS linker and by using the restriction enzyme BamHI, the linker can become 45 bp shorter. The BamHI-linker is inspired by the article "Quantitative Understanding of the Energy Transfer between Fluorescent Proteins Connected via Flexible Peptide Liners" by T.H. Evers et al from 29 August 2006. [2]
Figure 2: Schematical overview of the expressed OmpX (1) with mNeonGreen (2) in the outer membrane of E.coli.
Sequence
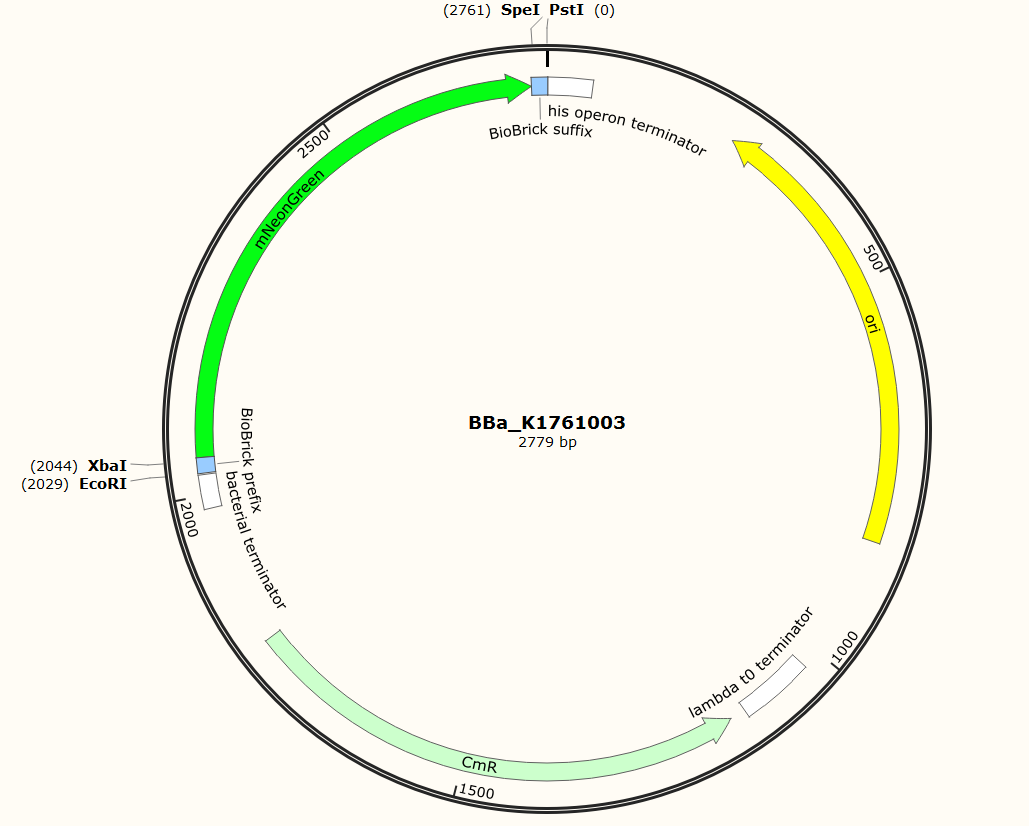
The sequence of our mTurquoise2 part (see Figure 3) has been verified by sequencing at StarSeq. It contains the prefix and suffix with the correct restriction sites (EcoRI, XbaI, SpeI and PstI). mNeonGreen is 711 bp long.
Figure 3: Snapgene plasmid overview of the BioBrick part BBa_K1761003. It shows the pSB1C3 vector with the prefix (containing the restriction sites EcoRI and XbaI), mNeonGreen and the suffix (containting the restiction sites SpeI and PstI).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization
The presence of mNeonGreen was tested with a fluorescence assay. This was done with the construct containing both OmpX and mNeonGreen and with mNeonGreen alone. For all the experiments we used the following vectors: pETDuet-1 with a construct inserted (OmpX + intracellular protein) or pSB1C3 with a strong promotor and mNeonGreen inserted, and pEVOL-pAzF (tRNA + tRNA synthetase). Both vectors were transformed into BL21(DE3). The expression was introduced by adding arabinose, IPTG and the unnatural amino acid.
Fluorescence Confirmation
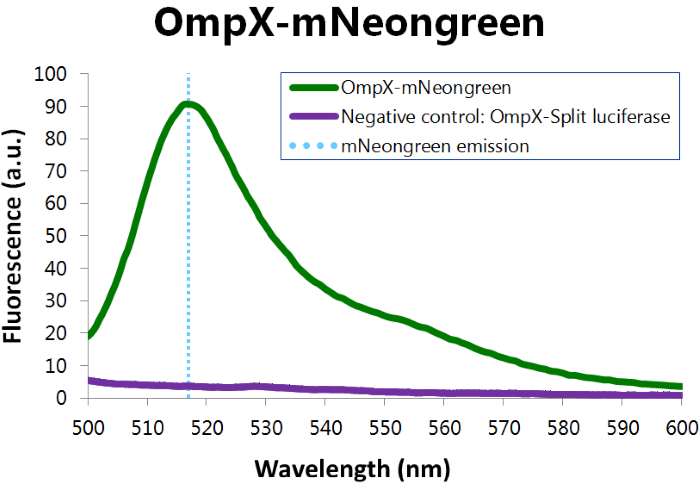
To confirm whether mNeonGreen is present in the bacteria, a fluorescence assay was performed. Excitation took place at a wavelength of 480 nm with a laser. Emission was read out at 517 nm. From this experiment, it can be concluded that mNeonGreen is present and works (see Figure 4). This was also tested with both OmpX – mNeonGreen and OmpX – NanoLuc inserted, which gave the same results (see Figure 5). For more information about how to perform a fluorescence assay, see our Protocol Page [http://2015.igem.org/Team:TU_Eindhoven/Project/Protocols].
Figure 4: Fluorescence results of OmpX - mNeonGreen
Figure 7: Fluorescence results of OmpX -mNeonGreen and OmpX - NanoLuc
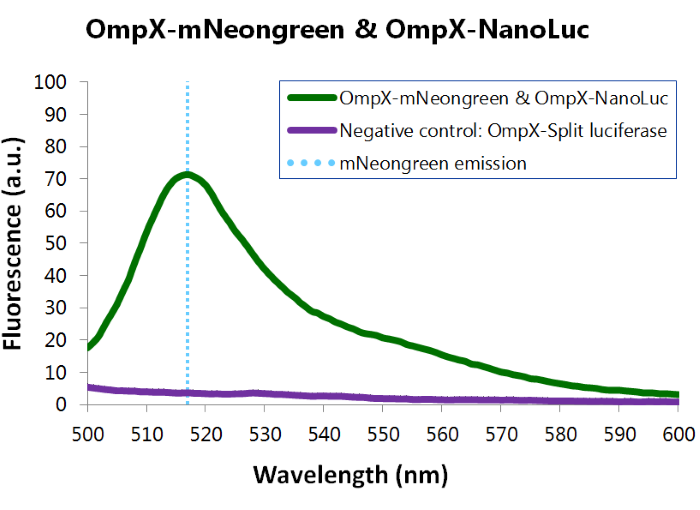
Bioluminescence Confirmation
To confirm whether mNeonGreen forms a good BRET (Bioluminescence resonance energy transfer) couple with NanoLuc, a bioluminescence measurement was performed (see Figure 8). In this experiment OmpX-NanoLuc's & OmpX-mNeonGreen's presence in the cells were tested by adding Nano-Glo substrate to the cells and measuring bioluminescence with the spectrophotometer. Figure 8 shows that OmpX-NanoLuc shows a peak characteristic for NanoLuc, indicating NanoLuc's presence within the cells. Moreover, the spectrogram shows a distinct shoulder near 517 nm, the emission wavelength of mNeongreen. Since no laser was used, excitation of mNeongreen can only be accomplished by NanoLuc so BRET (Bioluminescence resonance energy transfer) occured. This signal is measured when no click reaction is performed on the complex, meaning that this can be seen as the background noise of the sensor of iGEM team TU Eindhoven 2015. For more information about how to perform a bioluminescence measurement, see our Protocol Page [http://2015.igem.org/Team:TU_Eindhoven/Project/Protocols].
Figure 8: Bioluminiscence results NanoLuc and mNeonGreen.
References
[1] N.C. Shaner et al, “A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum.,” Nature Methods, vol. 10, no. 5, pp. 407-409, Mar. 2013.
[2] T.H. Evers et al, “Quantitative Understanding of the Energy Transfer between Fluorescent Proteins Connected via Flexible Peptide Linkers.,” Biochemistry, vol. 45, no. 44, pp. 13183-92, Nov. 2006.