Difference between revisions of "Part:BBa I715019"
Qiuxinyuan12 (Talk | contribs) |
Qiuxinyuan12 (Talk | contribs) (→Contribution: NUDT_CHINA 2015) |
||
| Line 19: | Line 19: | ||
==Contribution: NUDT_CHINA 2015== | ==Contribution: NUDT_CHINA 2015== | ||
| + | Auther: Xinyuan Qiu | ||
| + | Summary: In this contribution, we designed a pair of primers that can extent the usage of this part, and tested its function. | ||
===A pair of primers that can extent the usage of this part=== | ===A pair of primers that can extent the usage of this part=== | ||
Revision as of 13:05, 18 September 2015
Amino Half of GFP (aka GFP1)
A
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Contribution: NUDT_CHINA 2015
Auther: Xinyuan Qiu Summary: In this contribution, we designed a pair of primers that can extent the usage of this part, and tested its function.
A pair of primers that can extent the usage of this part
In our project, we plan to fuse the GFP1 to the C-terminal of TALE1 protein. However, the sample of BBa_ I715019 provided by the 2015 DNA distribution does not have a termination codon on its 3’ terminal to stop the translation. To fix this, we designed a pair of primers to add a termination codon on the 3’ terminal of BBa_ I715019 to further extend its usage.
F-Prime: 5’- GAATTCGCGGCCGCTTCTAGAATGC-3’
R-Prime: 5’- GGACTAGTATTATTGTTTGTCTGCC-3’
Functional Test
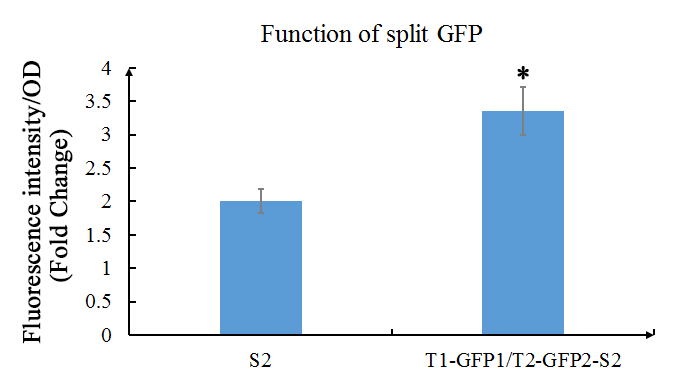
We also used the improved version (using the primers above) of GFP1 and GFP2 (that part is also improved) and tested the their functions. In our experiment, GFP1 was fused with TALE1, GFP2 was fused with TALE2; and SCAF2 was added into the plasmid. The plasmid was transferred into E.coli BL21(DE3) and cultured in LB with 30mg/ml Chloramphenicol to OD600=0.6, then inducted with 1mM IPTG overnight.
Fig. 1 Evaluation of the functions of split GFP. The green fluorescence (Ex: 488 nm; Em: 538 nm) of split GFP was detected after overnight culture of E.coli with or without GFP1/2 under the 1mM of IPTG induction. Relative fluorescence intensity was calculated with normalization of OD600 value. The relative fluorescence intensity of S2 control group was set arbitrarily at 1.0, and the levels of other groups were adjusted correspondingly. This experiment was run in three parallel reactions, and the data represent results obtained from at least three independent experiments. *0.01<p<0.05.
The results shows that the existence of GFP1 and GFP2 can observably increase the fluorescent intensity. Which then indicates that these two parts work as expected.