Difference between revisions of "Part:BBa K1824006"
(→Characterization) |
(→Characterization) |
||
| Line 10: | Line 10: | ||
===Characterization=== | ===Characterization=== | ||
[[File:T7.jpg]] | [[File:T7.jpg]] | ||
| + | <br>'''Figure 2:''' The fluorescence of T7+A1 with different dilution ratio at 30℃ and 37℃(Control and IPTG). | ||
| + | <br> | ||
| + | <br> | ||
| + | <br>eGFP protein was used as report protein to show the function of ribothermometer. The bacteria were centrifuged and observed under UV light by eyes directly. Then the bacteria were sonicated and plate reader was used to measure the fluorescence of eGFP. | ||
| + | <br>Figure 2 shows the result of A1 ribothermometer with T7 promoter. It seems that A1 ribothermometer also works well with T7 promoter. When IPTG is added as the inducer, the fluorescence of the samples at 37℃ is 6 times higher than the fluorescence of the samples at 30℃. On the contrary, in the absence of IPTG the fluorescence of each sample is really low, which means the amount of protein express is low. | ||
Latest revision as of 13:14, 17 September 2015
Consensus Promoter T7 + RNA Thermometer A1
This is a composite regulatory part that consisting of T7 promoter BBa_I13453 and RNA Thermometer A1 BBa_K1824558 for temperature induced gene expression at 42 degrees. The possible secondary structure of A1 was simulated by RNAstructure (Fig.1). This combination is compatible with the assembly method that XJTLU-CHINA promoted.
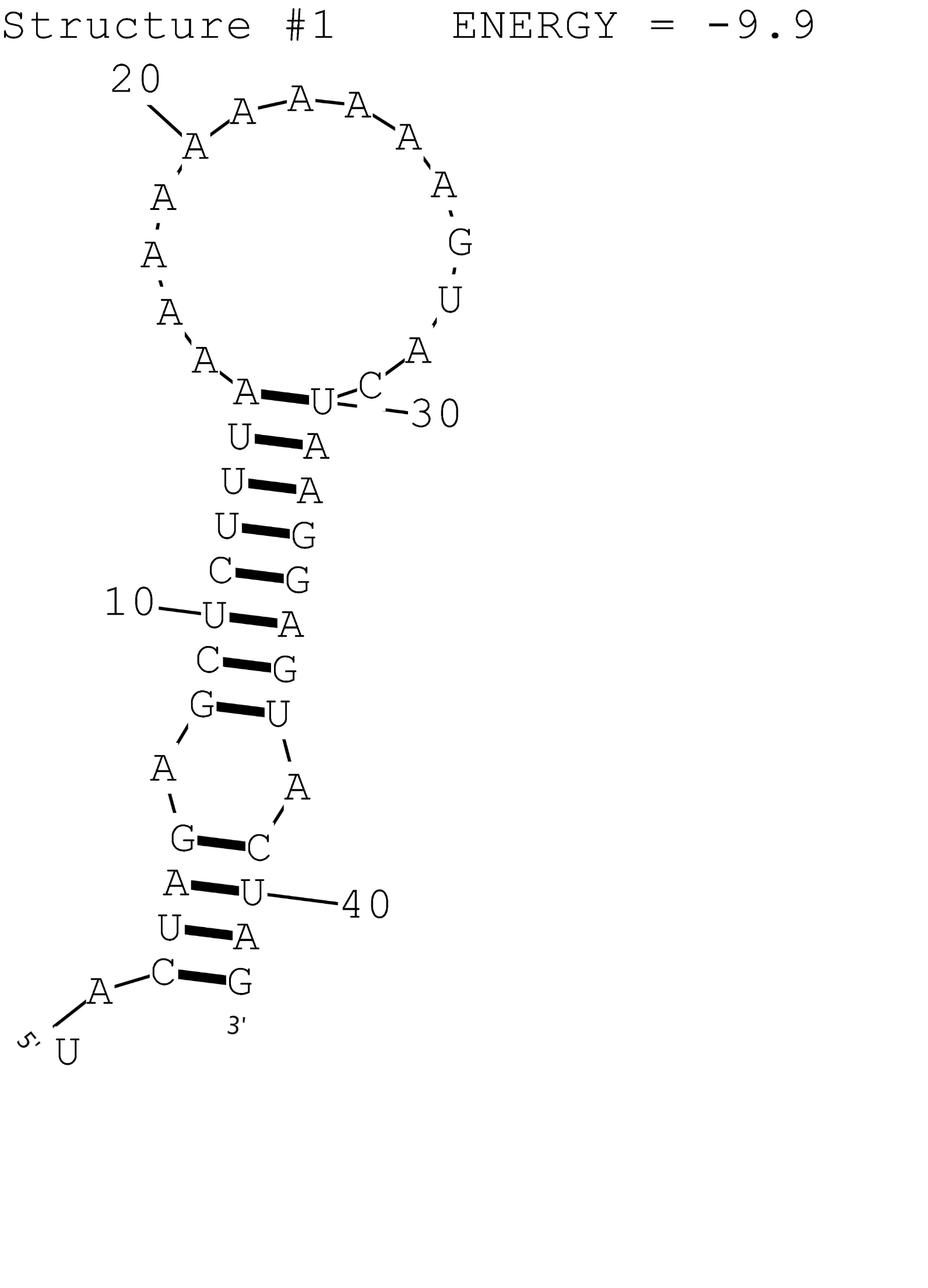
Figure 1: Possible secondary structure of RNAT A1.
Characterization

Figure 2: The fluorescence of T7+A1 with different dilution ratio at 30℃ and 37℃(Control and IPTG).
eGFP protein was used as report protein to show the function of ribothermometer. The bacteria were centrifuged and observed under UV light by eyes directly. Then the bacteria were sonicated and plate reader was used to measure the fluorescence of eGFP.
Figure 2 shows the result of A1 ribothermometer with T7 promoter. It seems that A1 ribothermometer also works well with T7 promoter. When IPTG is added as the inducer, the fluorescence of the samples at 37℃ is 6 times higher than the fluorescence of the samples at 30℃. On the contrary, in the absence of IPTG the fluorescence of each sample is really low, which means the amount of protein express is low.
