Difference between revisions of "Part:BBa K1720003"
(→Vector Map:) |
|||
| Line 29: | Line 29: | ||
===Vector Map:=== | ===Vector Map:=== | ||
[[File:SCUT2015_China_shRNA1_vector.png|800px|thumb|left|]] | [[File:SCUT2015_China_shRNA1_vector.png|800px|thumb|left|]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
===Vector Components:=== | ===Vector Components:=== | ||
[[File:SCUT2015_China_Vector component PDE5A1.png|900px|thumb|left|]] | [[File:SCUT2015_China_Vector component PDE5A1.png|900px|thumb|left|]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
Revision as of 12:43, 5 September 2015
Human phosphodiesterase 5A gene silencing device NO.1
This device is uesd for silencing the human phosphodiesterase 5A (PDE5A) gene.A U6 promoter driving a designed, synthetic shRNA-like miRNA followed by the terminator.
PDE5A is a cGMP-binding, cGMP-specific phosphodiesterase, a member of the cyclic nucleotide phosphodiesterase family. This phosphodiesterase specifically hydrolyzes cGMP to 5'-GMP. It is involved in the regulation of intracellular concentrations of cyclic nucleotides and is important for smooth muscle relaxation in the cardiovascular system.
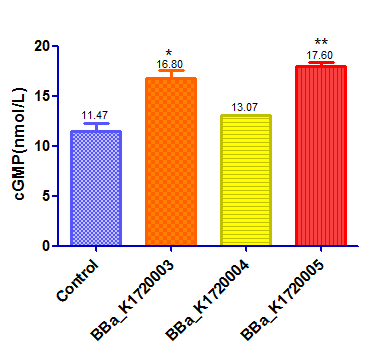
We designed 3 silencing device and test their function at the same time.Here is the another two device :BBa_K1720004,BBaK172005
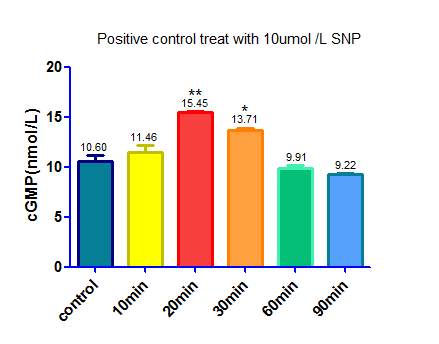
This device with a GFP reporter was then transfected into HEK293 cells by lentiviral vector.Once we silence the PDE5A gene the level of cGMP will be up regulated as a result. The positive control was HEK293 cells that treat with Sodium Nitroprusside ,a NO donator that activate sGC and up regulate the level of cGMP. A negative control was made by transfecting an empty vector that does not contain scilencing device. We used Elisa to detect cGMP level. The results are as follow:
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 273
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 247
- 1000COMPATIBLE WITH RFC[1000]
Vector Map:
Vector Components:
Virus Titer: (3.23±2)×10^8 TU/ml
Funtional titer is determined based on q-PCR amplification of a small fragment from the lentiviral vector-WRPE that is integrated into the genome of transduced 293T cells.
Experiment 1:
At the beginning of our experiment, we aimed to prove that HEK293 cells can be transfected by our vector. In our vector we inserted EGFP gene as a repoter.Once HEK293 cells are transfected successfully green fluorescence signal will be observed under fluorescence microscope.
Protocol: 1. Seed cells to be 40% confluent at a 35mm culture dish.
2. Dilute 10ul lentiviral vector in 1ml DMEM medium containing 10% FBS
3. Withdraw culture medium from 35mm culture dish.
4. Add vector-DMEM complex to cells
5. Incubate for 15 hours.
6. Withdraw vector-DMEM complex from culture dish.
7. Add 2ml DMEM medium containing 10% FBS to cells and incubate for 10 hours
8. Observe the cells under Inverted fluorescence microscope.
Result:
From the picture we can see that vivo green fluorescence signal was observed which indicated that HEK293 cells had been transfected successfully!
Experiment 2:
After we proved that HEK293 cells can be transfected, PDE5A gene expression levels were determined by real-time PCR.
Protocol:
Result:
Experiment 3:
After we scilencing the PDE5A gene,we used cGMP Elisa kit to detect the cGMP concentration to see whether cGMP concentration can be up regulated by our scilencing device.
Protocol:
1. Prepare all standards and samples be added in duplicate to the micro elisa stripplate.
2. Add standard : Set Standard wells , testing sample wells. Add standard 50 μl to standard well .
3. Add testing sample 10 μl then add Sample Diluent 40 μl to testing sample well (samples were 5 times diluted ) ; Blank well doesn’t add anyting.
4. Add 100 μl of HRP-conjugate reagent to each well , cover with an adhesive strip and incubate for 60 minutes at 37°C.
5. Aspirate each well and wash by filling each well with Wash Solution (400μl ), repeating the process four times for a total of five washes. After the last wash, remove any remaining Wash Solution by decanting. Invert the plate and blot it against clean paper towels.
6. Add chromogen solution A 50μl and chromogen solution B 50μl to each well.Gently mix and incubate for 15 minutes at 37 C . Protect from light .
7. Add 50μl Stop Solution to each well.
8. Read the Optical Density ( O . D .) at 450 nm using a Microplate Reader.
Note:
1.Standard ( S0 → S5 ) concentration was followed by: 0,2,4,8,16,32 nmol/L.
2.We used BCA protein assay kit to detect total protein level as a internal reference and the result was corrected by it.
Result: