Difference between revisions of "Part:BBa K1554003"
(→Usage and Biology) |
|||
| Line 10: | Line 10: | ||
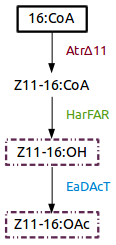
[https://parts.igem.org/Part:BBa_K1554001 Part:BBa_K1554001] (AtrΔ11), [https://parts.igem.org/Part:BBa_K1554002 Part:BBa_K1554002] (HarFAR) and [https://parts.igem.org/Part:BBa_K1554003 Part:BBa_K1554003] (EaDAcT) are enzymes of a biosynthesis pathway that lead to the production of insect sexual pheromones, Z11-16:OH and Z11-16:OAc, using palmitate as substrate. | [https://parts.igem.org/Part:BBa_K1554001 Part:BBa_K1554001] (AtrΔ11), [https://parts.igem.org/Part:BBa_K1554002 Part:BBa_K1554002] (HarFAR) and [https://parts.igem.org/Part:BBa_K1554003 Part:BBa_K1554003] (EaDAcT) are enzymes of a biosynthesis pathway that lead to the production of insect sexual pheromones, Z11-16:OH and Z11-16:OAc, using palmitate as substrate. | ||
| − | + | ||
| + | [[File:VUPV_pathway.png]] | ||
| + | |||
Figure 1. Insect sexual pheromone pathway for ''Nicotiana benthamiana''. | Figure 1. Insect sexual pheromone pathway for ''Nicotiana benthamiana''. | ||
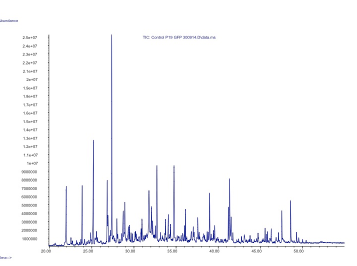
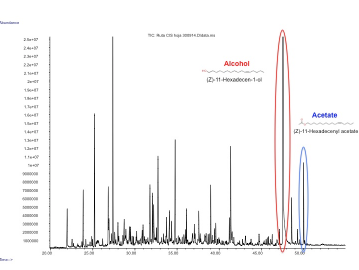
In our project we made a device with these three pheromones and expressed them by transient expression it in our plant chasis, ''Nicotiana benthamiana''. In order to check if the insect sexual pheromones were present, we performed the analysis using HS-SPME coupled to GC-MS. We observed to additional peaks in the transformed plants that were not present in the control and have a similar mass spectrum and retention time as the standards, which confirmed that both molecules were the desired pheromones, (Z)-11-hexadecen-1-ol and (Z)-11-hexadecenyl acetate. | In our project we made a device with these three pheromones and expressed them by transient expression it in our plant chasis, ''Nicotiana benthamiana''. In order to check if the insect sexual pheromones were present, we performed the analysis using HS-SPME coupled to GC-MS. We observed to additional peaks in the transformed plants that were not present in the control and have a similar mass spectrum and retention time as the standards, which confirmed that both molecules were the desired pheromones, (Z)-11-hexadecen-1-ol and (Z)-11-hexadecenyl acetate. | ||
| − | + | [[File:UPV_rutas-biosintesis_feromonas.png]] | |
| − | Figure 2. GC-MS analysis of the volatile organic compounds from a negative control of Nicotiana benthamiana. | + | |
| + | Figure 2. GC-MS analysis of the volatile organic compounds from a negative control of ''Nicotiana benthamiana''. | ||
| + | |||
| + | [[File:VUPV_pheromone.png]] | ||
| − | + | Figure 3. GC-MS analysis of the volatile organic compounds from a genetically engineered ''Nicotiana benthamiana'' to produce insect pheromones. | |
| − | Figure 3. GC-MS analysis of the volatile organic compounds from a genetically engineered Nicotiana benthamiana to produce insect pheromones. | + | |
Revision as of 23:35, 15 October 2014
EaDAcT
EaDAcT is a diacylglycerol acetyltransferase from the plant Euonymus alatus.
Usage and Biology
The EaDAcT protein is a diacylglycerol acetyltransferase coming from Euonymus alatus which transforms fatty alcohols into fatty aldehides. Our team used it in with Part:BBa_K1554001 and Part:BBa_K1554002 to produce the insect pheromone Z11-16:OAc in Nicotiana benthamiana using palmitate:CoA as precursor. Its role is to convert Z11-16:OH into Z11-16:OAc.
Part:BBa_K1554001 (AtrΔ11), Part:BBa_K1554002 (HarFAR) and Part:BBa_K1554003 (EaDAcT) are enzymes of a biosynthesis pathway that lead to the production of insect sexual pheromones, Z11-16:OH and Z11-16:OAc, using palmitate as substrate.
Figure 1. Insect sexual pheromone pathway for Nicotiana benthamiana.
In our project we made a device with these three pheromones and expressed them by transient expression it in our plant chasis, Nicotiana benthamiana. In order to check if the insect sexual pheromones were present, we performed the analysis using HS-SPME coupled to GC-MS. We observed to additional peaks in the transformed plants that were not present in the control and have a similar mass spectrum and retention time as the standards, which confirmed that both molecules were the desired pheromones, (Z)-11-hexadecen-1-ol and (Z)-11-hexadecenyl acetate.
Figure 2. GC-MS analysis of the volatile organic compounds from a negative control of Nicotiana benthamiana.
Figure 3. GC-MS analysis of the volatile organic compounds from a genetically engineered Nicotiana benthamiana to produce insect pheromones.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 883
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]