Difference between revisions of "Part:BBa K1399001"
m |
|||
| Line 6: | Line 6: | ||
LVA tag is commonly found attached to repressors in various gene networks (e.g. oscillators). | LVA tag is commonly found attached to repressors in various gene networks (e.g. oscillators). | ||
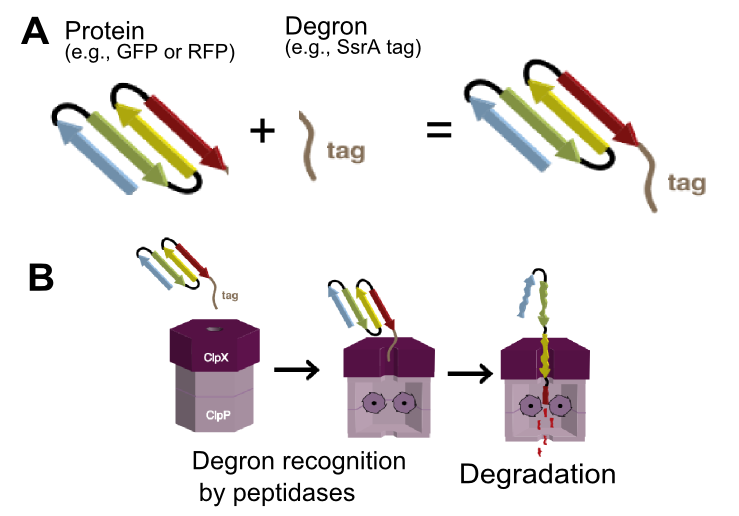
The tag encodes peptide sequence AANDENYALVA and is recognized by ClpA and ClpX unfoldases and ClpX mediator SspB.[1] ClpA and ClpX then form a proteosome-like complex with ClpP protease and the protein is degraded (Figure 1).[1] | The tag encodes peptide sequence AANDENYALVA and is recognized by ClpA and ClpX unfoldases and ClpX mediator SspB.[1] ClpA and ClpX then form a proteosome-like complex with ClpP protease and the protein is degraded (Figure 1).[1] | ||
| − | [[File:EDiGEM14-SsrA degron mediated degradation.png|300px|thumb|right|'''Figure 1 SsrA degron mediated protein degradation.''' ('''A''') Any version of SsrA tags can be attached to any protein of interest (e.g. RFP or GFP). ('''B''') The tag is recognized by ClpX | + | [[File:EDiGEM14-SsrA degron mediated degradation.png|300px|thumb|right|'''Figure 1 SsrA degron mediated protein degradation.''' ('''A''') Any version of SsrA tags can be attached to any protein of interest (e.g. RFP or GFP). ('''B''') The tag is recognized by ClpX unfoldase forming a complex with ClpP protease and the tagged protein gets degraded.]] |
The final three residues of the tag determines the strength of interaction with ClpX and thus the final protein degradation rate.[2] The LVA tag is reported to lead to fast protein degradation, degrading GFP with rate -0.018 per minute.[2] However, be aware that exact protein degradation rate depends on multiple factors: ClpXP and ClpAP protease and SspB mediator concentrations, protein stability, Km of binding to the protease, temperature [3]. | The final three residues of the tag determines the strength of interaction with ClpX and thus the final protein degradation rate.[2] The LVA tag is reported to lead to fast protein degradation, degrading GFP with rate -0.018 per minute.[2] However, be aware that exact protein degradation rate depends on multiple factors: ClpXP and ClpAP protease and SspB mediator concentrations, protein stability, Km of binding to the protease, temperature [3]. | ||
Revision as of 22:22, 15 October 2014
RFP from Discosoma striata (coral) with LVA-ssrA degradation tag
Mutant RFP from Discosoma striata (coral) (Part:BBa_E1010) with added LVA-ssrA degradation tag. The tag increases RFP turn-over rate, thus providing better temporal resolution of red fluorescence. In the same time, maximal fluorescence amplitudes will be lower as newly formed protein is degraded as soon as it is formed. LVA tag is commonly found attached to repressors in various gene networks (e.g. oscillators). The tag encodes peptide sequence AANDENYALVA and is recognized by ClpA and ClpX unfoldases and ClpX mediator SspB.[1] ClpA and ClpX then form a proteosome-like complex with ClpP protease and the protein is degraded (Figure 1).[1]
The final three residues of the tag determines the strength of interaction with ClpX and thus the final protein degradation rate.[2] The LVA tag is reported to lead to fast protein degradation, degrading GFP with rate -0.018 per minute.[2] However, be aware that exact protein degradation rate depends on multiple factors: ClpXP and ClpAP protease and SspB mediator concentrations, protein stability, Km of binding to the protease, temperature [3].
References: [1] Flynn, J. M. et al. Overlapping recognition determinants within the ssrA degradation tag allow modulation of proteolysis. Proc. Natl. Acad. Sci. U. S. A. 98, 10584–9 (2001). [2] Andersen, J. B. et al. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl. Environ. Microbiol. 64, 2240–6 (1998). [3] Purcell, O., Grierson, C. S., Bernardo, M. Di & Savery, N. J. Temperature dependence of ssrA-tag mediated protein degradation. J. Biol. Eng. 6, 10 (2012).
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 555
Illegal AgeI site found at 667 - 1000COMPATIBLE WITH RFC[1000]