Difference between revisions of "Part:BBa K1420005"
| Line 16: | Line 16: | ||
| − | <b> | + | <b> Protein </b> |
| − | + | MerT is a 116-residue (12.4 kDa) transmembrane protein. The exact structure isn't known, but the protein is predicted to have three transmembrane helices with a cysteine pair accessible from the periplasmic side and another pair on the cytoplasmic side. | |
<b> Mechanism </b> | <b> Mechanism </b> | ||
| − | The transport mechanism of ionic mercury from the bacterial cell periplasm to cytosplasm is not completely understood. | + | The transport mechanism of ionic mercury from the bacterial cell periplasm to cytosplasm is not completely understood. Hg(II) is transferred from the periplasmic cysteine pair to the cytoplasmic pair, where it may go directly to MerA or transfer to cytoplasmic low-molecular mass thiols. |
| + | |||
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
Revision as of 03:37, 13 October 2014
merT, mercuric transport protein
Overview
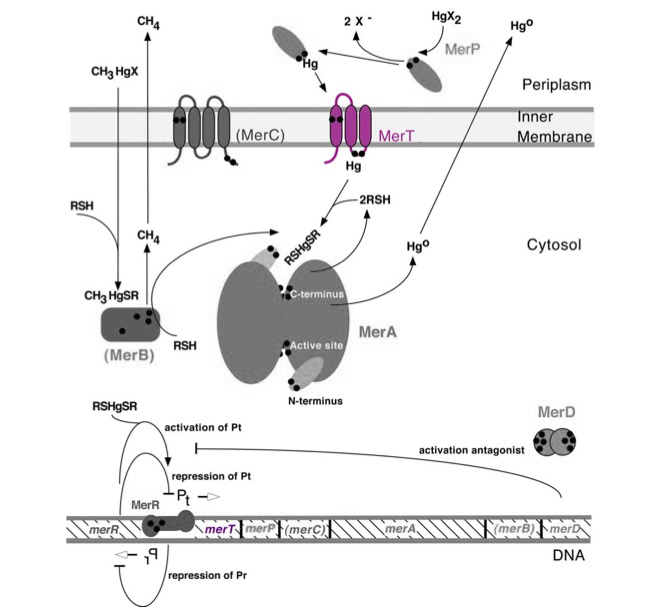
Transmembrane mercuric binding gene merT (0.3Kb) encodes MerT, which transports Hg(II) species from the periplasm through the membrane. MerT and gene merT are highlighted in purple in Figure 1. The lower part of the Figure 1 shows the arrangement of mer genes in the operon, and merT is located upstream of merP, another gene that encodes a mercury transport protein.
Figure 1. Model of transmembrane mercuric binding protein MerT. The symbol • indicates a cysteine residue. RSH indicates cytosolic thiol redox buffers such as glutathione. Figure 1 shows the interactions of MerT, in purple, with mercury compounds and other gene products of mer operon. (This figure is adapted from "Bacterial mercury resistance from atoms to ecosystems". Reference: T. Barkay et al. FEMS Microbiology Reviews 27 (2003) 355-384.)
merT is found in the Serratia marcescens plasmid pDU1358 on the other side of the bidirectional promoter opposite of merR. It is 351 base pairs long and it codes for a mercuric transport protein.
Protein
MerT is a 116-residue (12.4 kDa) transmembrane protein. The exact structure isn't known, but the protein is predicted to have three transmembrane helices with a cysteine pair accessible from the periplasmic side and another pair on the cytoplasmic side.
Mechanism
The transport mechanism of ionic mercury from the bacterial cell periplasm to cytosplasm is not completely understood. Hg(II) is transferred from the periplasmic cysteine pair to the cytoplasmic pair, where it may go directly to MerA or transfer to cytoplasmic low-molecular mass thiols.
Usage and Biology
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 45
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI site found at 30