Difference between revisions of "Part:BBa K1420003"
(→Overview) |
(→Overview) |
||
| Line 17: | Line 17: | ||
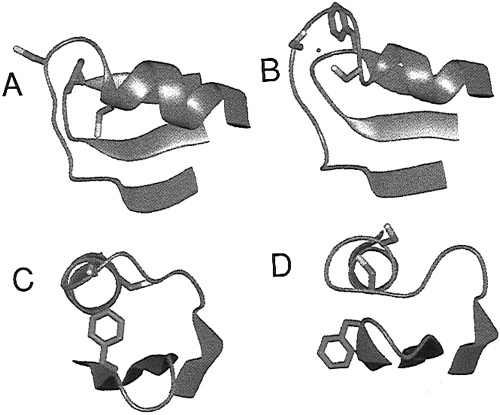
'''Figure 2.''' Structure of MerP as determined by NMR studies. A shows a reduced MerP as viewed from above. B shows a MerP binding a Hg(II) ion, showing the two cysteine residues that become surface exposed. C and D show the protein as viewed down a helix. Reference: ''R. Steele et. al.'', "Structures of the Reduced and Mercury-Bound Forms of MerP, the Periplasmic Protein from the Bacterial Mercury Detoxification System". Biochemistry, 1997, 36 (23), pp 6885–6895. | '''Figure 2.''' Structure of MerP as determined by NMR studies. A shows a reduced MerP as viewed from above. B shows a MerP binding a Hg(II) ion, showing the two cysteine residues that become surface exposed. C and D show the protein as viewed down a helix. Reference: ''R. Steele et. al.'', "Structures of the Reduced and Mercury-Bound Forms of MerP, the Periplasmic Protein from the Bacterial Mercury Detoxification System". Biochemistry, 1997, 36 (23), pp 6885–6895. | ||
| − | |||
| − | |||
| − | In the periplasm, MerP competes with thiol-mediated redox proteins for Hg(II). Like periplasmic nutrient transporters, MerP is the most abundantly synthesized subunit in its operon due to its role in periplasmic Hg(II) transport. | + | MerP is not essential for Hg(II) uptake, as MerT can import Hg(II) by itself. Additionally, there is the possibility of a Cys14Ser mutation on MerP which blocks Hg(II) uptake by MerT. If ''merP'' is expressed alone, it will exist in an oxidized state which is less stable than the reduced state it is found in when expressed as part of the greater ''mer'' operon. In the periplasm, MerP competes with thiol-mediated redox proteins for Hg(II). Like periplasmic nutrient transporters, MerP is the most abundantly synthesized subunit in its operon due to its role in periplasmic Hg(II) transport. |
[[File:MNiGEM_ZOI_MerP.jpg|left|400px|x]] | [[File:MNiGEM_ZOI_MerP.jpg|left|400px|x]] | ||
Revision as of 03:28, 13 October 2014
MerP, mercuric transport protein periplasmic component
Overview
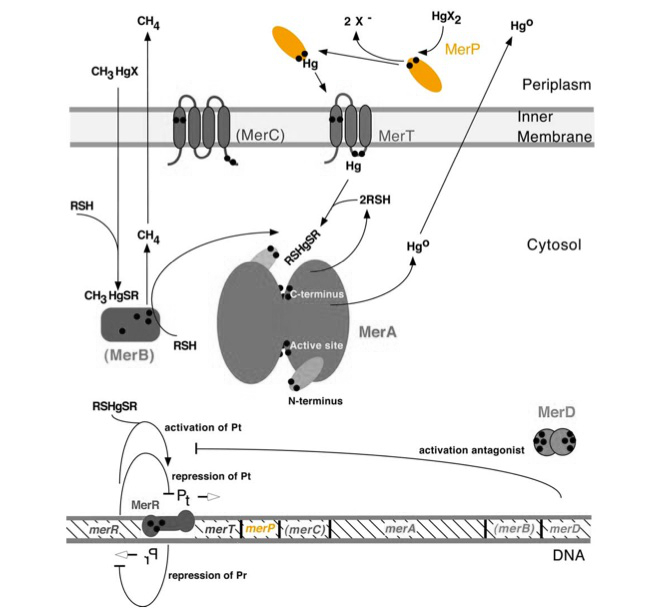
merP (0.3Kb) encodes a periplasmic transport protein. MerP and gene merP are highlighted in orange in Figure 1. The lower part of the Figure 1 shows the arrangement of mer genes in the operon, and merP is located downstream of merT, another gene that encodes a mercury transport protein.
Figure 1. Model of mercury resistance operon. The symbol • indicates a cysteine residue. RSH indicates cytosolic thiol redox buffers such as glutathione. Figure 1 shows the interactions of MerP, in orange, with mercury compounds and other gene products of mer operon. (This figure is adapted from "Bacterial mercury resistance from atoms to ecosystems". Reference: T. Barkay et al. FEMS Microbiology Reviews 27 (2003) 355-384.)
MerP is a 72 amino acid periplasmic transport protein. MerP functions as a monomer with two alpha helices overlaying a four-strand antiparallel beta sheet. As shown in Figure 2, MerP binds a single Hg(II) ion at two conserved cysteine residues, these cysteines define the metal binding motif of MerP. Similar motifs are also found in other proteins that are involved in the transportation of thiophilic metal cations. The MerP cysteine residues uptake a Hg(II) ion and remove any attached ligands before passing the ion to the MerT transmembrane protein.
Figure 2. Structure of MerP as determined by NMR studies. A shows a reduced MerP as viewed from above. B shows a MerP binding a Hg(II) ion, showing the two cysteine residues that become surface exposed. C and D show the protein as viewed down a helix. Reference: R. Steele et. al., "Structures of the Reduced and Mercury-Bound Forms of MerP, the Periplasmic Protein from the Bacterial Mercury Detoxification System". Biochemistry, 1997, 36 (23), pp 6885–6895.
MerP is not essential for Hg(II) uptake, as MerT can import Hg(II) by itself. Additionally, there is the possibility of a Cys14Ser mutation on MerP which blocks Hg(II) uptake by MerT. If merP is expressed alone, it will exist in an oxidized state which is less stable than the reduced state it is found in when expressed as part of the greater mer operon. In the periplasm, MerP competes with thiol-mediated redox proteins for Hg(II). Like periplasmic nutrient transporters, MerP is the most abundantly synthesized subunit in its operon due to its role in periplasmic Hg(II) transport.
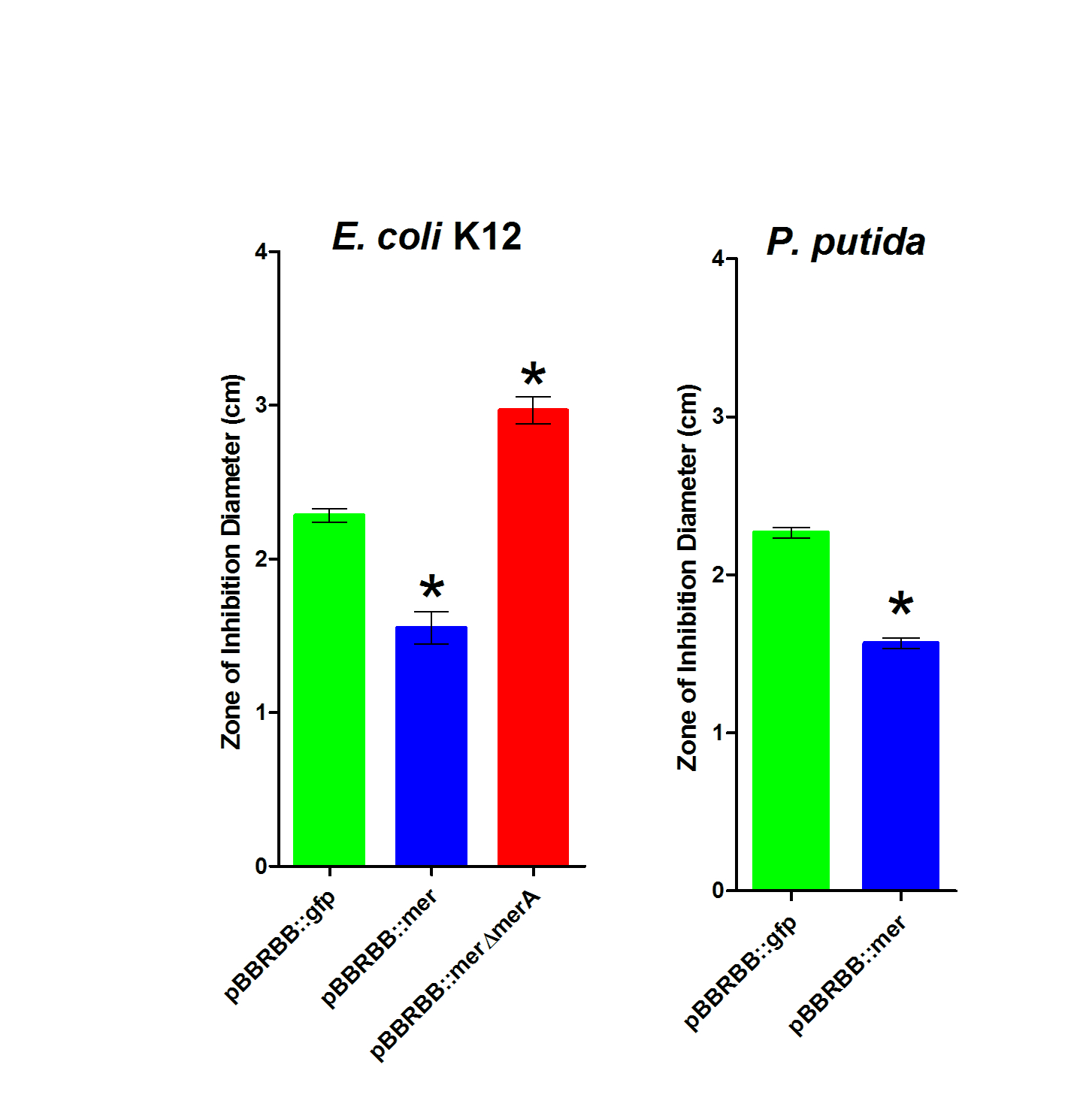
For the zone of inhibition studies, 10µL of 0.1M HgCl2 were spotted on a filter disk in the middle of an agar plate. The diameter of the Zone of Inhibition was measured in triplicate. In the linked figure, P. putida shows a smaller zone of inhibition for the strain with the Mer operon, as expected. In the E. coli K12 sample, the same relationship between the strain with the Mer operon and the empty vector was observed. In addition, vector with MerA deleted has a higher zone of inhibition, which would be expected as the bacteria would be unable to reduce Hg(II) and remove it, leading to a toxic bioaccumulation that kills the bacteria.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]