Difference between revisions of "Part:BBa K1379000"
(→Reference) |
(→Reference) |
||
| Line 48: | Line 48: | ||
Rhodius V., Segall-Shapiro T., Sharon B., Ghodasara A., Orlova E., Tabakh H., . . . Voigt C. (2013). ''Design of orthogonal genetic switches based on a crosstalk map of σs, anti-σs, and promoters.'' Molecular Systhetic Biology .doi:10.1038/msb.2013.58 | Rhodius V., Segall-Shapiro T., Sharon B., Ghodasara A., Orlova E., Tabakh H., . . . Voigt C. (2013). ''Design of orthogonal genetic switches based on a crosstalk map of σs, anti-σs, and promoters.'' Molecular Systhetic Biology .doi:10.1038/msb.2013.58 | ||
<br><br> | <br><br> | ||
| − | J. R. Kelly, A. J. Rubin, J. H. Davis, J. Cumbers, M. J. Czar, ..., D. Endy. (2009). Measuring the activity of BioBrick promoters using an in vivo reference standard. Journal of Biological Engineering, 3, 4. doi: 10.1186/1754-1611-3-4 | + | J. R. Kelly, A. J. Rubin, J. H. Davis, J. Cumbers, M. J. Czar, ..., D. Endy. (2009). Measuring the activity of BioBrick promoters using an in vivo reference standard. Journal of Biological Engineering, 3, 4. doi: 10.1186/1754-1611-3-4 |
Revision as of 21:20, 11 October 2014
PcelA
Usage and Biology
PcelA (or celAp) is a σX regulated promoter from Streptococcus pneumoniae. It is a member of the Cin-Box or Com-Box promoter family that share the 8 base pair consensus sequence of TACGAATA, where the sigma factor σX BBa_K1379004 (or ComX) binds to and promotes transcription initiation. (Piotrowski, Luo, & Morrison, 2009) During the exponential growth of S. pneumoniae, Competence Signal Peptide (CSP) mediated quorum sensing induces expression of σX. σX as a global regulator then directs S. pneumoniae to enter a transient competent cell state. PcelA, alongside with other Com-Box promoters, is turned on by σX and drives expression of the competence protein CelA. (BioCyc)
Voigt and his colleagues have demonstrated that orthogonal gene expression could be achieved through the use of σs, anti-σs and synthetic promoters. (Rhodius et al., 2013). This principle has been demonstrated to work in E. coli (see characterization below), in which PcelA can only be turned on in the presence of σX.
iGEM 2014 Hong_Kong_HKUST Team has cloned PcelA from S. pneumoniae strain NCTC7465 and characterized its Relative Promoter Units (RPU) in presence and absence of a σX generator BBa_K1379006. Promoter PcomFA (BBa_K1379001), which is another Com-Box promoter recognized by σX, has also been characterized.
The transcription start site of this promoter has yet to be experimentally located.
Characterization
For characterization, PcelA promoter was assembled with the promoter measurement kit BBa_E0240 to give the PcelA Measurement Kit BBa_K1379002 in plasmid pSB3K3. The construct was further assembled with σX generator BBa_K1379006 to give BBa_K1379005.
Qualitative characterization was performed by comparing intensities of GFP signals from colonies of E. coli DH10B strain holding the PcelA Measurement Kits with and without the σX generator under a fluorescent macroscope with UV filter. Measurement kit for standard reference promoter BBa_J23101, BBa_I20260 was used as a positive control; BBa_E0240 was used as the negative control for background fluorescence.
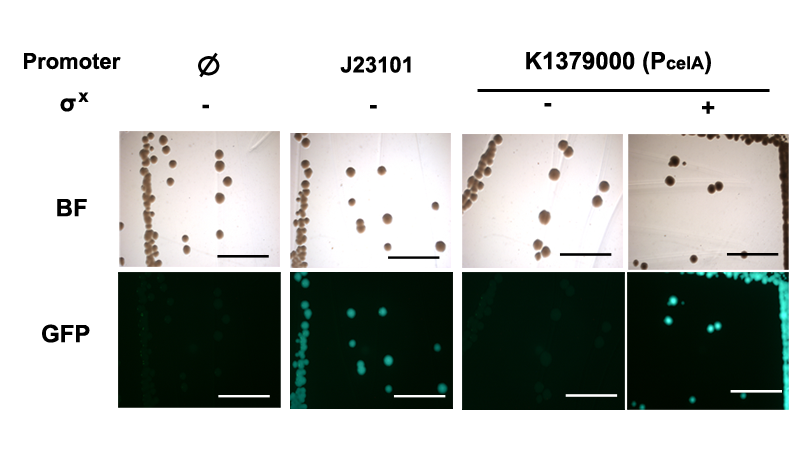
Quantitative characterization was performed following the protocol described in “Measuring the activity of BioBrick promoters using an in vivo reference standard” (Kelly et al., 2009). E. coli DH10B strains holding the constructs with or without σX generator respectively were grown to mid-log phases. GFP intensities and cell densities were then sampled every 30 minutes for 5 consecutive time points to obtain growth rates and GFP synthesis rates. The GFP synthesis rates were then compared to that of standard reference promoter BBa_J23101 measurement device BBa_I20260 to obtain the Relative Promoter Units (RPUs). For subtraction of background fluorescence, pSB3K3 holding BBa_E0240 was measured alongside. The measurement was done with 3 replicas.
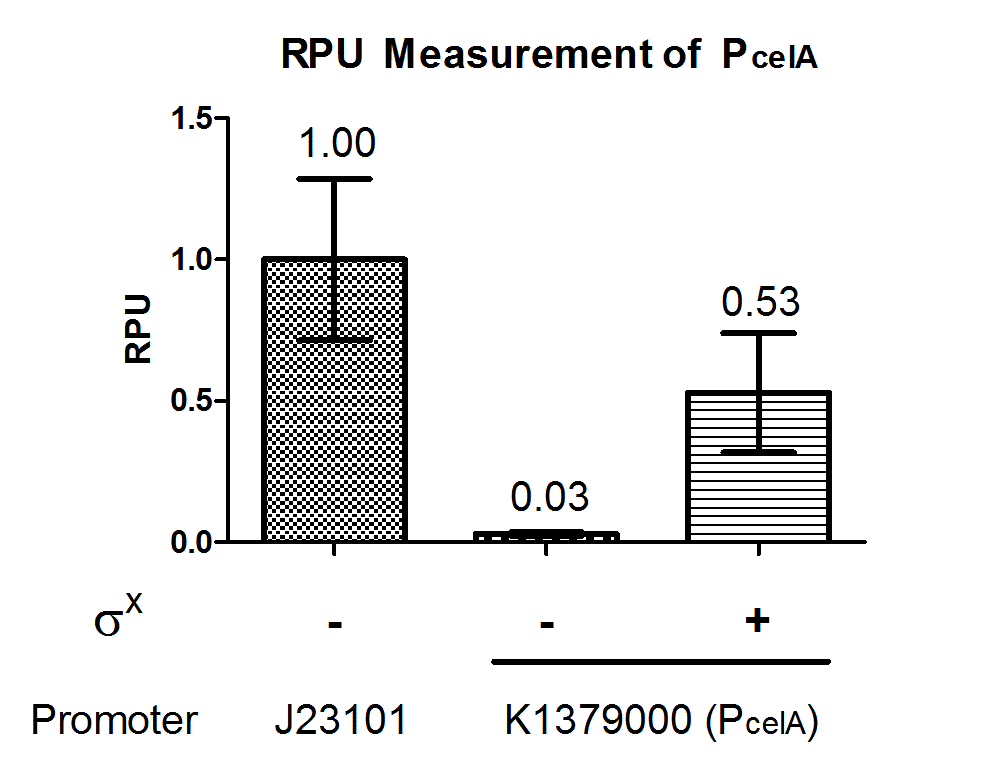
The above results showed that PcelA is functional in E. coli and is turned on specifically in presence of σX. Further characterization can be done to evaluate crosstalk between this sigma factor-promoter pair and their other counterparts.
Extensive documentation of this characterization, including the wet lab protocols and data processing, can be found in [http://2014.igem.org/Team:Hong_Kong_HKUST/pneumosensor/characterization iGEM HKUST 2014 Wiki Page].
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Reference
BioCyc was retrieved from http://www.biocyc.org/SPNE171101/NEW-IMAGE?type=GENE&object=GJC8-867
Luo P., & Morrison D. (2003). Transient Association of an Alternative Sigma Factor, ComX, with RNA Polymerase during the Period of Competence for Genetic Transformation in Streptococcus pneumoniae. Journal of Bacteriology. doi:10.1128/JB.185.1.349-358.2003
Piotrowski A., Luo P., & Morrison D. (2009). Competence for genetic transformation in Streptococcus pneumoniae: termination of activity of the alternative sigma factor ComX is independent of proteolysis of ComX and ComW. Journal of Bacteriology. doi:10.1128/JB.01750-08
Rhodius V., Segall-Shapiro T., Sharon B., Ghodasara A., Orlova E., Tabakh H., . . . Voigt C. (2013). Design of orthogonal genetic switches based on a crosstalk map of σs, anti-σs, and promoters. Molecular Systhetic Biology .doi:10.1038/msb.2013.58
J. R. Kelly, A. J. Rubin, J. H. Davis, J. Cumbers, M. J. Czar, ..., D. Endy. (2009). Measuring the activity of BioBrick promoters using an in vivo reference standard. Journal of Biological Engineering, 3, 4. doi: 10.1186/1754-1611-3-4
