Difference between revisions of "Part:BBa K1152013"
(→Usage and Biology) |
|||
| Line 25: | Line 25: | ||
<!-- --> | <!-- --> | ||
| − | Figure 1: Restriction digest of BBa_K1152013 with EcoRI, XbaI, SpeI and PstI (construct marked with red circle) confirms correct cloning of the part into pSB1C3. | + | '''Figure 1''': Restriction digest of BBa_K1152013 with EcoRI, XbaI, SpeI and PstI (construct marked with red circle) confirms correct cloning of the part into pSB1C3. |
| + | |||
| + | <br> | ||
| + | <br> | ||
We tested the IndC expression contruct by cotransformation in different E. coli strains and in combination with different PPTase expression constructs (Figure 2). | We tested the IndC expression contruct by cotransformation in different E. coli strains and in combination with different PPTase expression constructs (Figure 2). | ||
| Line 31: | Line 34: | ||
[[image:Heidelberg_IndPD_Fig6.png|400px]] | [[image:Heidelberg_IndPD_Fig6.png|400px]] | ||
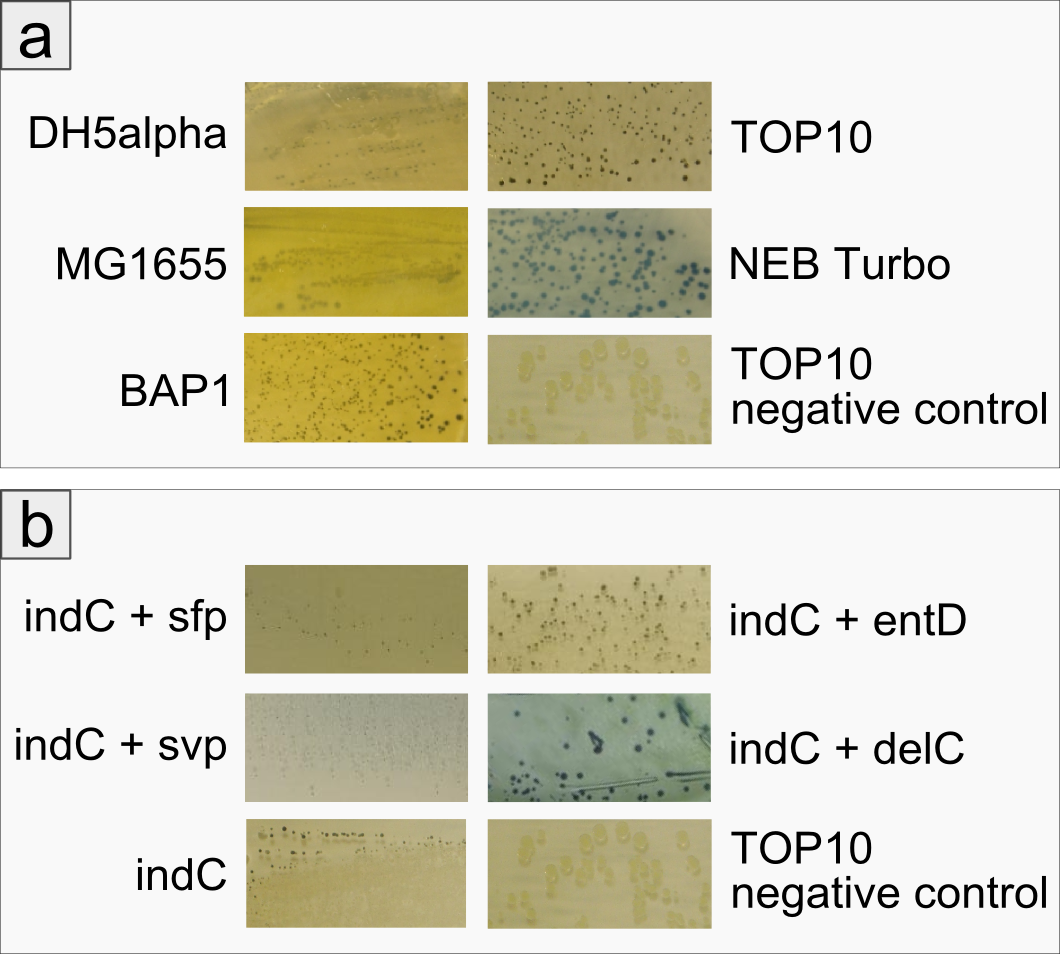
| − | Figure 2: Comparison between different E. coli strains and PPTases: a) Comparison of different E. coli strains examining growth and indigoidine production The figure shows five different strains of E. coli that have been co-transformed with an indC expression plasmid and a sfp expression plasmid. The negative control is E. coli TOP10 without a plasmid. All transformants have been grown on LB agar for 48 hours at room temperature, cells were not induced. One can see that even without induction all strains express the indigoidine synthetase and produce the blue pigment indigoidine. However, the strains BAP1 and NEB Turbo grow faster in the first day, exhibiting a white phenotype (data not shown). Colonies on the plate of E. coli TOP10 are very small and dark blue/ black. Assuming that indigoidine production inhibits cell growth due to its toxicity, we concluded that TOP10 produced the most indigoidine among the strains we tested. We used E. coli TOP10 for the following experiments. b) Comparison between different PPTases concerning overall indigoidine production The Figure shows ''E. coli'' TOP10 cells co-transformed with indC and four different PPTases (sfp, svp, entD and delC), respectively. The image bottom left shows ''E. coli'' TOP10 cells without additional PPTase and the negative control is TOP10 without a plasmid. | + | '''Figure 2''': Comparison between different E. coli strains and PPTases: a) Comparison of different E. coli strains examining growth and indigoidine production The figure shows five different strains of E. coli that have been co-transformed with an indC expression plasmid and a sfp expression plasmid. The negative control is E. coli TOP10 without a plasmid. All transformants have been grown on LB agar for 48 hours at room temperature, cells were not induced. One can see that even without induction all strains express the indigoidine synthetase and produce the blue pigment indigoidine. However, the strains BAP1 and NEB Turbo grow faster in the first day, exhibiting a white phenotype (data not shown). Colonies on the plate of E. coli TOP10 are very small and dark blue/ black. Assuming that indigoidine production inhibits cell growth due to its toxicity, we concluded that TOP10 produced the most indigoidine among the strains we tested. We used E. coli TOP10 for the following experiments. b) Comparison between different PPTases concerning overall indigoidine production The Figure shows ''E. coli'' TOP10 cells co-transformed with indC and four different PPTases (sfp, svp, entD and delC), respectively. The image bottom left shows ''E. coli'' TOP10 cells without additional PPTase and the negative control is TOP10 without a plasmid. |
Revision as of 03:26, 5 October 2013
IndC Indigoidine Synthetase device
This part encodes the non-ribosomal peptide synthetase that synthesizes the blue pigment indigoidine. The IndC gene was amplified from P. luminescens laumondii TT01 (DSM15139) and cloned into pSB1C3.
Usage and Biology
The single module NRPS indC from P. luminescens contains an internal oxidation domain (Ox-domain) in its A-domain and a special TE-domain . This enzyme first adenylates L-glutamine (A-domain), which is then attached to the T-domain. The TE-domain cleaves and catalyzes the cyclization of the substrate, which is further oxidized by the Ox-domain. The oxidation of two cyclic glutamines results in the formation of the insoluble blue pigment indigoidine.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 4087
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1467
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 2730
Successful cloning of the IndC expression device was confirmed by restriction digested (Figure 1) followed by sequencing.
Figure 1: Restriction digest of BBa_K1152013 with EcoRI, XbaI, SpeI and PstI (construct marked with red circle) confirms correct cloning of the part into pSB1C3.
We tested the IndC expression contruct by cotransformation in different E. coli strains and in combination with different PPTase expression constructs (Figure 2).
Figure 2: Comparison between different E. coli strains and PPTases: a) Comparison of different E. coli strains examining growth and indigoidine production The figure shows five different strains of E. coli that have been co-transformed with an indC expression plasmid and a sfp expression plasmid. The negative control is E. coli TOP10 without a plasmid. All transformants have been grown on LB agar for 48 hours at room temperature, cells were not induced. One can see that even without induction all strains express the indigoidine synthetase and produce the blue pigment indigoidine. However, the strains BAP1 and NEB Turbo grow faster in the first day, exhibiting a white phenotype (data not shown). Colonies on the plate of E. coli TOP10 are very small and dark blue/ black. Assuming that indigoidine production inhibits cell growth due to its toxicity, we concluded that TOP10 produced the most indigoidine among the strains we tested. We used E. coli TOP10 for the following experiments. b) Comparison between different PPTases concerning overall indigoidine production The Figure shows E. coli TOP10 cells co-transformed with indC and four different PPTases (sfp, svp, entD and delC), respectively. The image bottom left shows E. coli TOP10 cells without additional PPTase and the negative control is TOP10 without a plasmid.