Difference between revisions of "Part:BBa K1150024"
| Line 50: | Line 50: | ||
==Proof of Function== | ==Proof of Function== | ||
After knowing, that the protein is expressed functional tests were performed. As stated before, when targeted to an open, endogenous locus we expect change of the epigenetic state to repression. To test this, the<b> endogenous VEGF-A locus</b> in HEK293-T cells was chosen, as it is well-characterized and open in HEK293T cells. Additionally it can easily be measured by ELISA analysis. <br> | After knowing, that the protein is expressed functional tests were performed. As stated before, when targeted to an open, endogenous locus we expect change of the epigenetic state to repression. To test this, the<b> endogenous VEGF-A locus</b> in HEK293-T cells was chosen, as it is well-characterized and open in HEK293T cells. Additionally it can easily be measured by ELISA analysis. <br> | ||
| − | Cells were seeded into 24-well format and transfected with the plasmid and different crRNAs, coded by derviative plasmids of the | + | Cells were seeded into 24-well format and transfected with the plasmid and different crRNAs, coded by derviative plasmids of the [https://parts.igem.org/Part:BBa_K1150034 RNAimer], that code for loci on the VEGF gene. These were: [https://parts.igem.org/Part:BBa_K1150036 -8], [https://parts.igem.org/Part:BBa_K1150037 -573], [https://parts.igem.org/Part:BBa_K1150038 +434], [https://parts.igem.org/Part:BBa_K1150039 -475] and [https://parts.igem.org/Part:BBa_K1150043 -573/+434]. As an off-target control the plasmid coding for the crRNA [https://parts.igem.org/Part:BBa_K1150035 EMX] was used. One control does not contain any target RNA. As an <b>internal standard</b> a constitutive SEAP reporter was used. After 24 hours, VEGF levels and SEAP levels were measured and the ratio was calculated. Error bars represent standard deviation. |
[1]Wolffe, A., et al. (1999). Epigenetics: Regulation Through Repression. Science 286169, 481. <br> | [1]Wolffe, A., et al. (1999). Epigenetics: Regulation Through Repression. Science 286169, 481. <br> | ||
Revision as of 22:26, 1 October 2013
uniCAS Histone Modifier (CMV promoter)
| pCMV:HA-NLS-dCas9-G9a-NLS:tBGH | |
|---|---|
| Function | DNA binding protein fused to a methyl histone transferase |
| Use in | Mammalian cells |
| RFC standard | RFC 25 |
| Backbone | pSB1C3 |
| Organism | Streptococcus pyogenes, Mus musculus |
| Source | Feng Zhang, Addgene Albert Jeltsch, University of Stuttgart |
| Submitted by | [http://2013.igem.org/Team:Freiburg Freiburg 2013] |
This device is combining the dCas9 protein, that enables multiple gene targeting with the set-domain of the murine G9a. dCas9 is working as a carrier for this histone methyltransferase and enables specific methylation of histone 3 Lysin 9 (H3K9me2/3) when targeted to a histone locus that is accessible for DNA binding proteins. Literature indicates that targeting the G9a Set-Domain to an open locus leads to a transcriptionally inactive state. [1] The usage of the strong CMV promoter enables this device to be expressed in a strong manner. If weaker expression levels are needed, we recommend using our uniCAS Histone Modificator device with an SV40 promoter (SV40:dCas9-G9a). For Western blot detection of our protein an HA-tag was fused to the protein. Additionally for the usage in mammalian system two NLS were fused to the protein to bring it to its site of action. The mRNA synthesis is stopped by an BGH terinator. Between dCas9 and the G9a Set-Domain a short linker was cloned to minimize disturbance between both proteins.
Usage and Biology
H3K9 methylation is a hallmark of repressed transcriptional states. [2] The murine G9a-Set domain is able to transfer methyl groups to H3K9 when targeting it to the DNA and repress transcription. G9a is also known to be involved in downstream signalling, but by targeting it to a specific locus we reduce the functionality to its histone modification ability. [3] The dCAS9 protein is able to be targeted to several loci at once as it interacts with small RNAs to build up a complex that will interact with complementary DNA strands. Its origin is the adaptive immune system of Streptococcus pyogenes called CRISPR. Hijacking this system leads to a whole new approach for multiple gene targeting. The team Freiburg 2013 combined these two elements to create a transcriptional repressor that is able to repress by a specific mechanism the targeted locus. This approach offers new possibilities for fundamental epigenetic research, tissue engineering and cancer research.
Functional tests
Expression
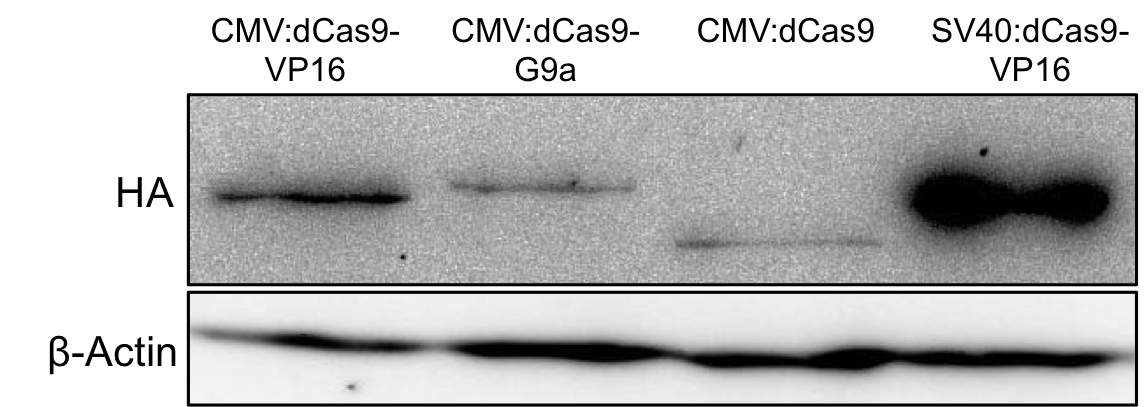
To make sure whether the protein is correctly expressed we performed Western blot analysis with an anti-HA antibody.
To do so HEK293T cells were seeded into 6-wells and transfected with the plasmid. After 48 hours of expression, cells were harvested, lysed and put on an SDS gel. After Western blot, a band is seen at the expected size and so we concluded that our protein is correctly expressed from the plasmid.
Figure 2.: HA-tag fused to different dCas9-fusion proteins encoded on the RFC25 pSB1C3 backbone.
Proof of Function
After knowing, that the protein is expressed functional tests were performed. As stated before, when targeted to an open, endogenous locus we expect change of the epigenetic state to repression. To test this, the endogenous VEGF-A locus in HEK293-T cells was chosen, as it is well-characterized and open in HEK293T cells. Additionally it can easily be measured by ELISA analysis.
Cells were seeded into 24-well format and transfected with the plasmid and different crRNAs, coded by derviative plasmids of the RNAimer, that code for loci on the VEGF gene. These were: -8, -573, +434, -475 and -573/+434. As an off-target control the plasmid coding for the crRNA EMX was used. One control does not contain any target RNA. As an internal standard a constitutive SEAP reporter was used. After 24 hours, VEGF levels and SEAP levels were measured and the ratio was calculated. Error bars represent standard deviation.
[1]Wolffe, A., et al. (1999). Epigenetics: Regulation Through Repression. Science 286169, 481.
[2]Snowden, A., et al. (2002). Gene-Specific Targeting of H3K9 Methylation Is Sufficient for Initiating Repression In Vivo. Current Biology 12, 2159-2166.
[3] Lee, D., et al. (2006). Histone 3 Lysine 9 Methyltransferase G9a Is a Transcriptional Coactivator for Nuclear Receptors. Journal of Biological Chemistry 281, 8476-8485.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 576
Illegal BglII site found at 900
Illegal BglII site found at 5375 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]