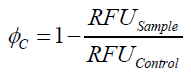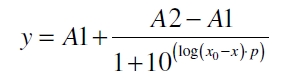Difference between revisions of "Part:BBa K525405"
(rollbackEdits.php mass rollback) |
|||
| Line 1: | Line 1: | ||
| − | + | __NOTOC__ | |
| + | <partinfo>BBa_K525405 short</partinfo> | ||
| + | |||
| + | [[Image:Bielefeld-Germany2011-S-Layer-Geometrien.jpg|thumb|300px|right]] | ||
| + | |||
| + | Fusion Protein of S-Layer SbpA and mCitrine | ||
| + | |||
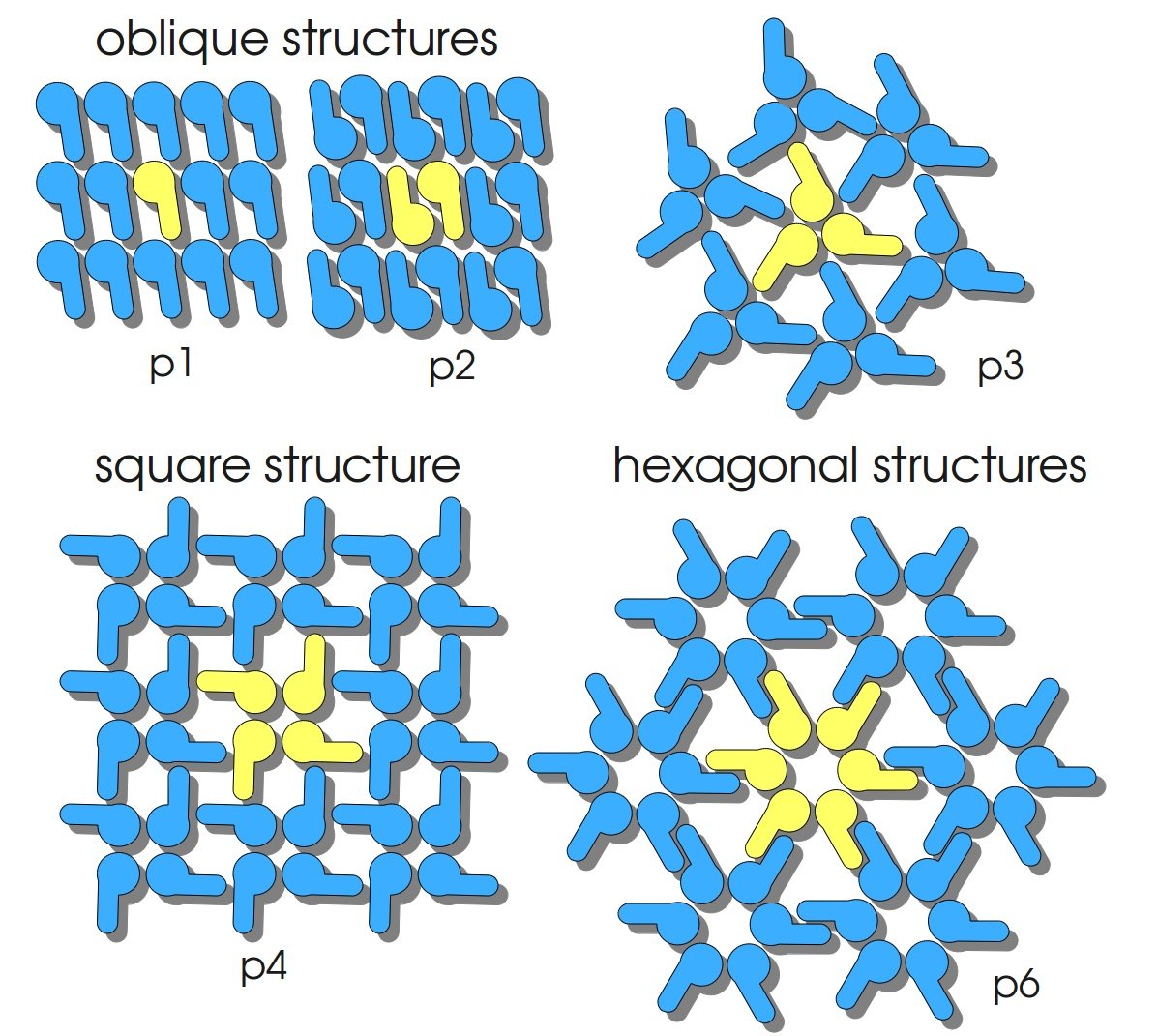
| + | S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S-layer geometry) it is possible to realize various practical applications ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr ''et al.'', 2007]). | ||
| + | |||
| + | |||
| + | ===Usage and Biology=== | ||
| + | |||
| + | S-layer proteins can be used as scaffold for nanobiotechnological applications and devices by e.g. fusing the S-layer's self-assembly domain to other functional protein domains. It is possible to coat surfaces and liposomes with S-layers. A big advantage of S-layers: after expressing in ''E. coli'' and purification, the nanobiotechnological system is cell-free. This enhances the biological security of a device. | ||
| + | |||
| + | This fluorescent S-layer fusion protein is used to characterize purification methods and the S-layer's ability to self-assemble on surfaces. It is also possible to use the characteristic of mCitrine as a pH indicator ([http://pubs.acs.org/doi/abs/10.1021/bm901071b Kainz ''et al.'', 2010]). | ||
| + | |||
| + | |||
| + | ===Important parameters=== | ||
| + | |||
| + | <center> | ||
| + | {|{{Table}} | ||
| + | !Experiment | ||
| + | !Characteristic | ||
| + | !Result | ||
| + | |- | ||
| + | |rowspan="6"|[[Part:BBa_K525405#Expression_in_E._coli | Expression (''E. coli'')]] | ||
| + | |Localisation | ||
| + | |Inclusion body | ||
| + | |- | ||
| + | |Compatibility | ||
| + | |''E. coli'' KRX and BL21(DE3) | ||
| + | |- | ||
| + | |Induction of expression | ||
| + | |Expression of T7 polymerase + IPTG, lactose | ||
| + | |- | ||
| + | |Inhibition of expression | ||
| + | |glucose | ||
| + | |- | ||
| + | |Specific growth rate (un-/induced) | ||
| + | |0.115 h<sup>-1</sup> / 0.077 h<sup>-1</sup> | ||
| + | |- | ||
| + | |Doubling time (un-/induced) | ||
| + | |6.02 h / 9.04 h | ||
| + | |- | ||
| + | |rowspan="3"|[[Part:BBa_K525405#Purification_of_SbpA_fusion_protein | Purification]] | ||
| + | |Molecular weight | ||
| + | |137.0 kDa | ||
| + | |- | ||
| + | |Theoretical pI | ||
| + | |4.93 | ||
| + | |- | ||
| + | |Excitation / emission | ||
| + | |515 / 529 nm | ||
| + | |- | ||
| + | |rowspan="3"|[[Part:BBa_K525405#Immobilization_behaviour | Immobilization behaviour]] | ||
| + | |[[Part:BBa_K525405#Optimal_bead_to_protein_ratio_for_immobilization | Saturation protein / bead ratio]] | ||
| + | |7 - 9 * 10<sup>-4</sup> | ||
| + | |- | ||
| + | |Immobilization time | ||
| + | |4 h | ||
| + | |- | ||
| + | |lattice structure | ||
| + | |oblique (p2) | ||
| + | |- | ||
| + | |} | ||
| + | </center> | ||
| + | |||
| + | |||
| + | <!-- --> | ||
| + | <span class='h3bb'>Sequence and Features</span> | ||
| + | <partinfo>BBa_K525405 SequenceAndFeatures</partinfo> | ||
| + | |||
| + | |||
| + | <!-- Uncomment this to enable Functional Parameter display | ||
| + | |||
| + | |||
| + | ===Functional Parameters=== | ||
| + | <partinfo>BBa_K525405 parameters</partinfo> | ||
| + | <!-- --> | ||
| + | |||
| + | ===Expression in ''E. coli''=== | ||
| + | |||
| + | The SbpA gene under the control of a T7 / lac promoter (<partinfo>K525403</partinfo>) was fused to mCitrine ([https://parts.igem.org/Part:BBa_J18931 BBa_J18931]) using Freiburg BioBrick assembly for characterization experiments. | ||
| + | |||
| + | The SbpA|mCitrine fusion protein was overexpressed in ''E. coli'' KRX after induction of T7 polymerase by supplementation of 0.1 % L-rhamnose and 1 mM IPTG using the autoinduction protocol by Promega. | ||
| + | |||
| + | [[Image:Bielefeld_2011_405_Growthcurve.png|600px|center|thumb| '''Figure 1: Growth curve of ''E. coli'' KRX expressing the fusion protein of SbpA and mCitrine with and without induction, cultivated at 37 °C in autoinduction medium with and without inductor, respectively. A curve depicting KRX wildtype is shown for comparison. After induction at approximately 4 h the OD<sub>600</sub> of the induced K525405 drops slightly when compared to the uninduced culture. Both cultures grow significantly slower than KRX wildtype.''']] | ||
| + | |||
| + | [[Image:Bielefeld_2011_405_RFU_OD.png|600px|center|thumb| '''Figure 2: RFU to OD<sub>600</sub> ratio of ''E. coli'' KRX expressing the fusion protein of SbpA and mCitrine with and without induction. A curve depicting KRX wildtype is shown for comparison. After induction at approximately 4 h the RFU to OD<sub>600</sub> ratio starts to rise in the induced culture. Compared to the uninduced culture the ratio is roughly three times higher. The KRX wildtype shows no variation in the RFU to OD<sub>600</sub> ratio.''']] | ||
| + | |||
| + | |||
| + | ===Purification of SbpA fusion protein=== | ||
| + | |||
| + | As seen in the analysis of the cultivations with expression of SbpA | mCitrine fusion proteins, these proteins form inclusion bodies in ''E. coli''. Inclusion bodies have the advantage that they are relatively easy to clean-up and are resistant to proteases. So the first purification step is to solve and set-free the inclusion bodies. This step is followed by two filtrations (300 kDa UF and 100 kDa DF/UF) to further concentrate and purify the S-layer proteins. After the filtrations, the remaining protein solution is dialyzed against ddH<sub>2</sub>O for 18 h at 4 °C in the dark. The dialysis leads to a precipitation of the water-insoluble proteins. After centrifugation of the dialysate the water-soluble S-layer monomers remain in the supernatant and can be used for recrystallization experiments. | ||
| + | |||
| + | [[Image:Bielefeld-Germany2011-305_405-Aufreinigung_symbol.png|800px|center]] | ||
| + | |||
| + | The fluorescence of some collected, important fractions of this purification strategy is shown in the following figure 3: | ||
| + | |||
| + | [[Image:Bielefeld-Germany2011-405-purificationfractions.jpg|700px|center|thumb|Fig. 3: '''Fluorescence of collected fractions during purification of <partinfo>K525405</partinfo> fusion protein. Abbreviations: pell.: pellet, s.n.: supernatant, ret.: retentate, perm.: permeate.''']] | ||
| + | |||
| + | A lot of protein is lost during the purification especially after centrifugation steps (compared to filtrations). The fluorescence in the urea containing fractions is lowered due to denaturation of the fluorescent protein. This purification strategy is very simple and can be carried out by nearly everyone in any lab being one first step to enable real do it yourself nanobiotechnology. | ||
| + | |||
| + | |||
| + | ===Alternative purification strategy=== | ||
| + | |||
| + | Sometimes the inclusion body purification does not give very clean fractions. That's why we developed another purification strategy for <partinfo>K525405</partinfo> monomers after inclusion body purification. This strategy consists of a capture with an IEX chromatography followed by a purification with HIC chromatography. Because of issues with the PES membranes we used to rebuffer our samples (see below - PES binds most of the S-layers) we lost a lot of our proteins in this step between the two chromatographies. So there was not enough protein left to stain it with Coomassie in a SDS-PAGE and time ran out to perform a silver staining. The fluorescence measurements of the collected fractions of the chromatographies are shown in the figures below. | ||
| + | |||
| + | [[Image:Bielefeld_Germany-405IEX.png|500px|thumb|center|'''Fig. 4: Fluorescence in the fractions of an anion exchange chromatography (IEX) with monomeric SbpA <html>|</html> mCitrine solution. A 1 mL HighTrap DEAE sepharose column by GE Healthcare was used for purification. The binding buffer (buffer A) contained 25 mM sodium chloride and the elution buffer (buffer B) 1 M sodium chloride (both 25 mM acetate buffer, pH 6.0). Abbreviations: FT = flow through, W = wash, E = elution. ''']] | ||
| + | |||
| + | [[Image:Bielefeld_Germany-405HIC.png|500px|thumb|center|'''Fig. 5: Fluorescence in the fractions of a hydrophobic interaction chromatography (HIC) with monomeric SbpA <html>|</html> mCitrine solution eluted from an IEX (see above). A 1 mL HighTrap butyl sepharose column by GE Healthcare was used for purification. The binding buffer (buffer A) contained 1200 mM ammonium sulfate and the elution buffer (buffer B) no ammonium sulfate (both 50 mM ammonium phosphate buffer, pH 7.0). Abbreviations: FT = flow through, E = elution. ''']] | ||
| + | |||
| + | ===Immobilization behaviour=== | ||
| + | |||
| + | After purification, solutions of monomeric SbpA S-layer proteins can be recrystallized and immobilized on silicon dioxide beads in recrystallization buffer (0.5 mM Tris-HCl, pH 9, 10 mM CaCl<sub>2</sub>). After the recrystallization procedure the beads are washed with and stored in ddH<sub>2</sub>O at 4 °C in the dark. The fluorescence of the collected fractions of recrystallization experiments with <partinfo>K525405</partinfo> are shown in Fig. 6. 100 mg beads were coated with 100 µg of protein. The figure shows, that not all of the protein is immobilized on the beads (supernatant fraction) but the immobilization is pretty stable (very low fluorescence in the wash). After the immobilization, the beads show a high fluorescence indicating the binding of the SbpA | mCitrine fusion protein. | ||
| + | |||
| + | [[Image:Bielefeld-Germany2011-405_100-fractions.jpg|600px|center|thumb|'''Fig. 6: Measured fluorescence of collected fractions of immobilization of purified <partinfo>K525405</partinfo> on silica dioxide beads (n = 3, 100 mg mL<sup>-1</sup> SiO<sub>2</sub>, time of recrystallization: 4 h). ''']] | ||
| + | |||
| + | |||
| + | ====Optimal bead to protein ratio for immobilization==== | ||
| + | |||
| + | To determine the optimal ratio of silica beads to protein for immobilization, the degree of clearance ϕ<sub>C</sub> in the supernatant is calculated and plotted against the concentration of silica beads used in the accordant immobilization experiment (compare Fig. 7): | ||
| + | |||
| + | |||
| + | [[Image:Bielefeld-Germany2011-degreeofclearanceformula.png|150px|center]] <div align="right">(1)</div> | ||
| + | |||
| + | |||
| + | The data was collected in three independent experiments. The fluorescence of the samples was measured in the supernatant of the immobilization experiment after centrifuging the silica beads. The fluorescence of the control was measured in a sample which was treated exactly like the others but no silica beads were added. 100 µg protein were used for one immobilization experiment. The data was fitted with a sigmoidal dose-response function of the form. | ||
| + | |||
| + | |||
| + | [[Image:Bielefeld_Doseresponse_fit.jpg|175px|center]] <div align="right">(2)</div> | ||
| + | |||
| + | |||
| + | with the Hill coefficient p, the bottom asymptote A1, the top asymptote A2 and the switch point log(x<sub>0</sub>) (R² = 0.997). | ||
| + | |||
| + | The fit indicates that a good silica concentration for 100 µg of protein is 200 - 250 mg mL<sup>-1</sup>. This set-up leads to saturated beads with low waste of protein. So a good protein / bead ratio to work with is 7 - 9 * 10<sup>-4</sup>. | ||
| + | |||
| + | |||
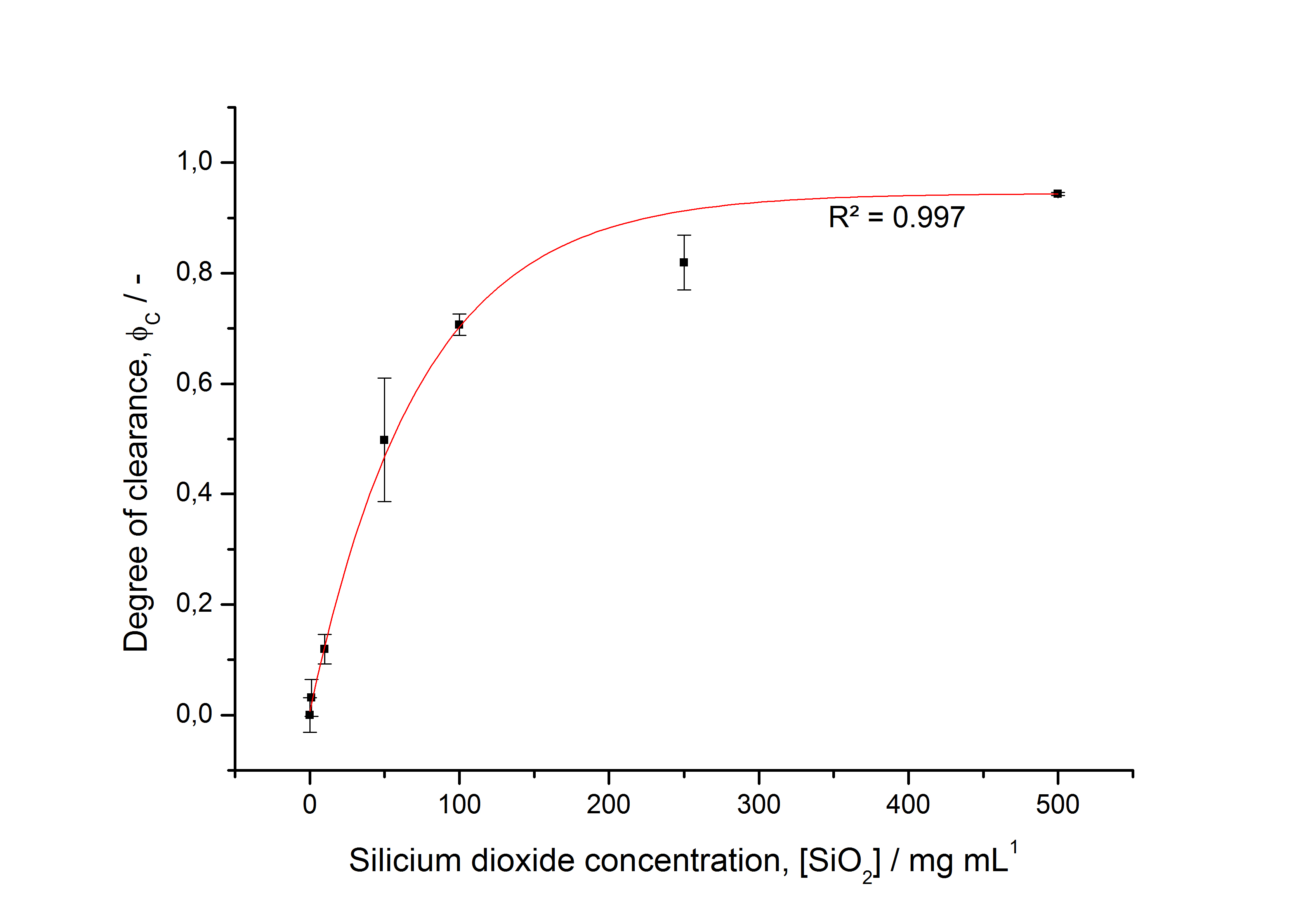
| + | [[Image:Bielefeld-Germany2011-degreeofclearance405.jpg|700px|center|thumb|'''Fig. 7: Degree of clearance of the fluorescence in the supernatant plotted against the concentration of silicon dioxide beads used to immobilize <partinfo>K525405</partinfo> (n = 3). Data is fitted with dose-reponse function (eq. (2), R² = 0.997). ''']] | ||
| + | |||
| + | |||
| + | ====Methods==== | ||
| + | |||
| + | '''Expression of S-layer genes in ''E. coli'' in shaking flasks''' | ||
| + | * Chassis: Promega's [http://www.promega.com/products/cloning-and-dna-markers/cloning-tools-and-competent-cells/bacterial-strains-and-competent-cells/single-step-_krx_-competent-cells/ ''E. coli'' KRX] | ||
| + | |||
| + | * Medium: LB medium supplemented with 20 mg L<sup>-1</sup> chloramphenicol | ||
| + | ** For autoinduction: Cultivations in LB-medium were supplemented with 0.1 % L-rhamnose and 1 mM IPTG as inducer and 0.05 % glucose | ||
| + | |||
| + | * cultivation: 37 °C with 120 rpm in shaking flasks | ||
| + | |||
| + | |||
| + | '''Production of SgsE''' | ||
| + | * Cultivation | ||
| + | ** Bioreactor: [http://www.bioengineering-inc.com/standard-reactors.php?id=2.1 Bioengineering NLF22 7 L] with Bioengineering DCU | ||
| + | ** Medium: [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Materials#HSG_medium HSG medium] with 20 mg L<sup>-1</sup> chloramphenicol | ||
| + | ** Culture volume: 4 L | ||
| + | ** Inoculation OD<sub>600</sub>: 0.2 | ||
| + | ** DO: 40 % airsaturation (controlled with stirrer cascade starting with 200 rpm) | ||
| + | ** pH: 7.0 (controlled with 20 % phosphoric acid and 2 M NaOH) | ||
| + | ** Induction after 4 h cultivation time with 2 % rhamnose and 0.1 mM IPTG (in culture medium) | ||
| + | ** Harvest after 13 h | ||
| + | |||
| + | *Cell lysis | ||
| + | ** Centrifuge down the cells (10000 g, 30 min, 4 °C) | ||
| + | ** Resuspend pellet in enzyme buffer | ||
| + | ** Cell lysis with high-pressure homogenizer (800 bar, 3 cycles at 4 °C) | ||
| + | ** Centrifuge down the lysate (10000 g, 60 min, 4 °C) | ||
| + | |||
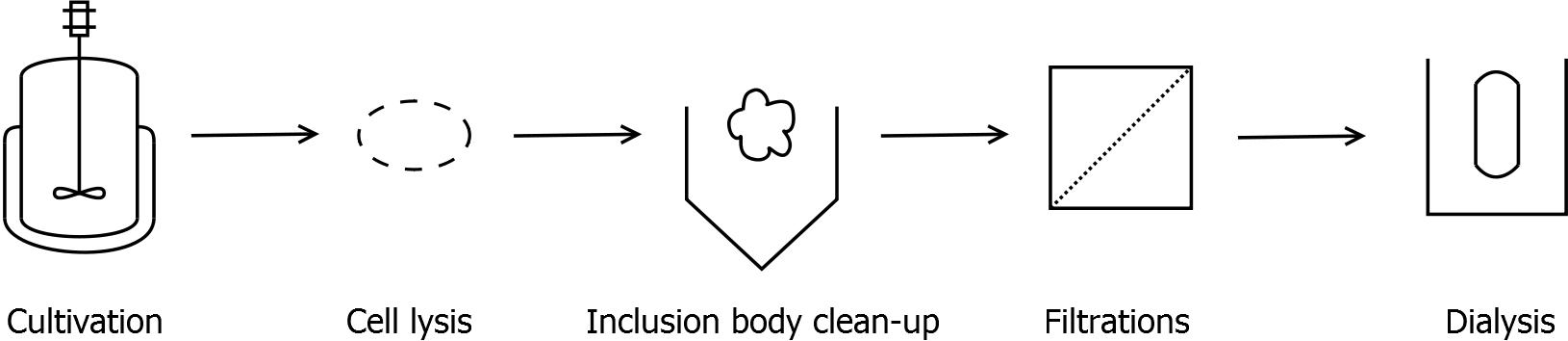
| + | *Inclusion body clean-up | ||
| + | ** wash pellet from cell lysis with water twice | ||
| + | ** after washing the pellet: incubate the pellet in [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Materials#Denaturation_buffer_for_inclusion_bodies denaturation buffer] for 60 min, 4 °C with vertical rotator | ||
| + | *** final concentration in denaturation buffer: 0.5 mg wet biomass per mL | ||
| + | ** centrifuge (60 min, >17000 g, 4 °C) | ||
| + | *** in general with all centrifugations during this clean-up: the higher the speed, the better the result | ||
| + | ** collect supernatant and incubate the pellet again in denaturation buffer (60 min, 4 °C, vertical rotator) | ||
| + | ** centrifuge (60 min, >17000 g, 4 °C) | ||
| + | ** collect supernatant and discard pellet | ||
| + | <html> | ||
| + | <div style="float:right; width:400px; text-align:center;"> | ||
| + | <img src="https://static.igem.org/mediawiki/igem.org/5/5f/Bielefeld-Germany2011-filtrationmodule.jpeg" width="90%" height="90%" /> | ||
| + | <br/> | ||
| + | </div> | ||
| + | </html> | ||
| + | *Filtration | ||
| + | ** Arrange the filtration module as shown on the right side. | ||
| + | ** Collect permeate of cross flow filtration with 300 kDa membrane of sample before ultrafiltration | ||
| + | *** This step is for removing cell debris | ||
| + | ** Diafiltrate with 100 kDa membrane against [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Materials#Denaturation_buffer_for_inclusion_bodies denaturation buffer] | ||
| + | *** constantly delute permeate with the buffer, keeping the permeate volume as low as possible | ||
| + | ** Used membranes: [http://www.millipore.com/catalogue/module/C7493 Milipore Pellicon XL 50] or XL 100 membranes | ||
| + | *** 50, 100 or 300 kDa cut-off | ||
| + | *** 50 cm<sup>2</sup> filtration area | ||
| + | *** tangential flow filter | ||
| + | *** Hydrophilic polyvinylidene fluoride membrane | ||
| + | ** Used pump: SciLog TANDEM 1081 peristaltic pump | ||
| + | *** flow rate during filtration: 40 mL min<sup>-1</sup> | ||
| + | |||
| + | * Dialysis | ||
| + | ** Fill retentate from DF/UF in dialysis tube ([http://www.carl-roth.de/ Roth], cellulose, 10 kDa cut-off) | ||
| + | ** Dialyse against ddH<sub>2</sub>O for 18 h at 4 °C in the dark | ||
| + | ** After dialysis: centrifuge down the precipitation (45 min, 17000 g, 4 °C) and collect the supernatant | ||
| + | ** Measure protein concentration in supernatant, dilute to 1 mg mL<sup>-1</sup> with ddH<sub>2</sub>O and store at 4 °C in the dark | ||
| + | |||
| + | |||
| + | '''Measuring of [https://parts.igem.org/Part:BBa_J18931 mCitrine]''' | ||
| + | * Take at least 500 µL sample for each measurement (200 µL is needed for one measurement) so you can perform a repeat determination | ||
| + | * Freeze biological samples at -80 °C for storage, keep cell-free at 4 °C in the dark | ||
| + | * To measure the samples thaw at room temperature and fill 200 µL of each sample in one well of a black, flat bottom 96 well microtiter plate (perform at least a repeat determination) | ||
| + | * Measure the fluorescence in a platereader (we used a [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader]) with following settings: | ||
| + | ** 20 sec orbital shaking (1 mm amplitude with a frequency of 87.6 rpm) | ||
| + | ** Measurement mode: Top | ||
| + | ** Excitation: 488 nm | ||
| + | ** Emission: 529 nm | ||
| + | ** Number of reads: 25 | ||
| + | ** Manual gain: 100 | ||
| + | ** Integration time: 20 µs | ||
| + | |||
| + | ===References=== | ||
| + | Kainz B, Steiner K, Möller M, Pum D, Schäffer C, Sleytr UB, Toca-Herrera JL (2010) Absorption, Steady-State Fluorescence, Fluorescence Lifetime, and 2D Self-Assembly Properties of Engineered Fluorescent S-Layer Fusion Proteins of ''Geobacillus stearothermophilus'' NRS 2004/3a, [http://pubs.acs.org/doi/abs/10.1021/bm901071b ''Biomacromolecules'' 11(1):207-214]. | ||
| + | |||
| + | Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM (2007) S-layers as a tool kit for nanobiotechnological applications, [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full ''FEMS Microbiol Lett'' 267(2):131-144]. | ||
Latest revision as of 16:17, 10 May 2013
Fusion Protein of S-Layer SbpA and mCitrine
Fusion Protein of S-Layer SbpA and mCitrine
S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S-layer geometry) it is possible to realize various practical applications ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr et al., 2007]).
Usage and Biology
S-layer proteins can be used as scaffold for nanobiotechnological applications and devices by e.g. fusing the S-layer's self-assembly domain to other functional protein domains. It is possible to coat surfaces and liposomes with S-layers. A big advantage of S-layers: after expressing in E. coli and purification, the nanobiotechnological system is cell-free. This enhances the biological security of a device.
This fluorescent S-layer fusion protein is used to characterize purification methods and the S-layer's ability to self-assemble on surfaces. It is also possible to use the characteristic of mCitrine as a pH indicator ([http://pubs.acs.org/doi/abs/10.1021/bm901071b Kainz et al., 2010]).
Important parameters
| Experiment | Characteristic | Result |
|---|---|---|
| Expression (E. coli) | Localisation | Inclusion body |
| Compatibility | E. coli KRX and BL21(DE3) | |
| Induction of expression | Expression of T7 polymerase + IPTG, lactose | |
| Inhibition of expression | glucose | |
| Specific growth rate (un-/induced) | 0.115 h-1 / 0.077 h-1 | |
| Doubling time (un-/induced) | 6.02 h / 9.04 h | |
| Purification | Molecular weight | 137.0 kDa |
| Theoretical pI | 4.93 | |
| Excitation / emission | 515 / 529 nm | |
| Immobilization behaviour | Saturation protein / bead ratio | 7 - 9 * 10-4 |
| Immobilization time | 4 h | |
| lattice structure | oblique (p2) |
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 104
Illegal BglII site found at 221
Illegal XhoI site found at 1996 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 76
Illegal AgeI site found at 3913 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 493
Illegal BsaI.rc site found at 622
Expression in E. coli
The SbpA gene under the control of a T7 / lac promoter (BBa_K525403) was fused to mCitrine (BBa_J18931) using Freiburg BioBrick assembly for characterization experiments.
The SbpA|mCitrine fusion protein was overexpressed in E. coli KRX after induction of T7 polymerase by supplementation of 0.1 % L-rhamnose and 1 mM IPTG using the autoinduction protocol by Promega.
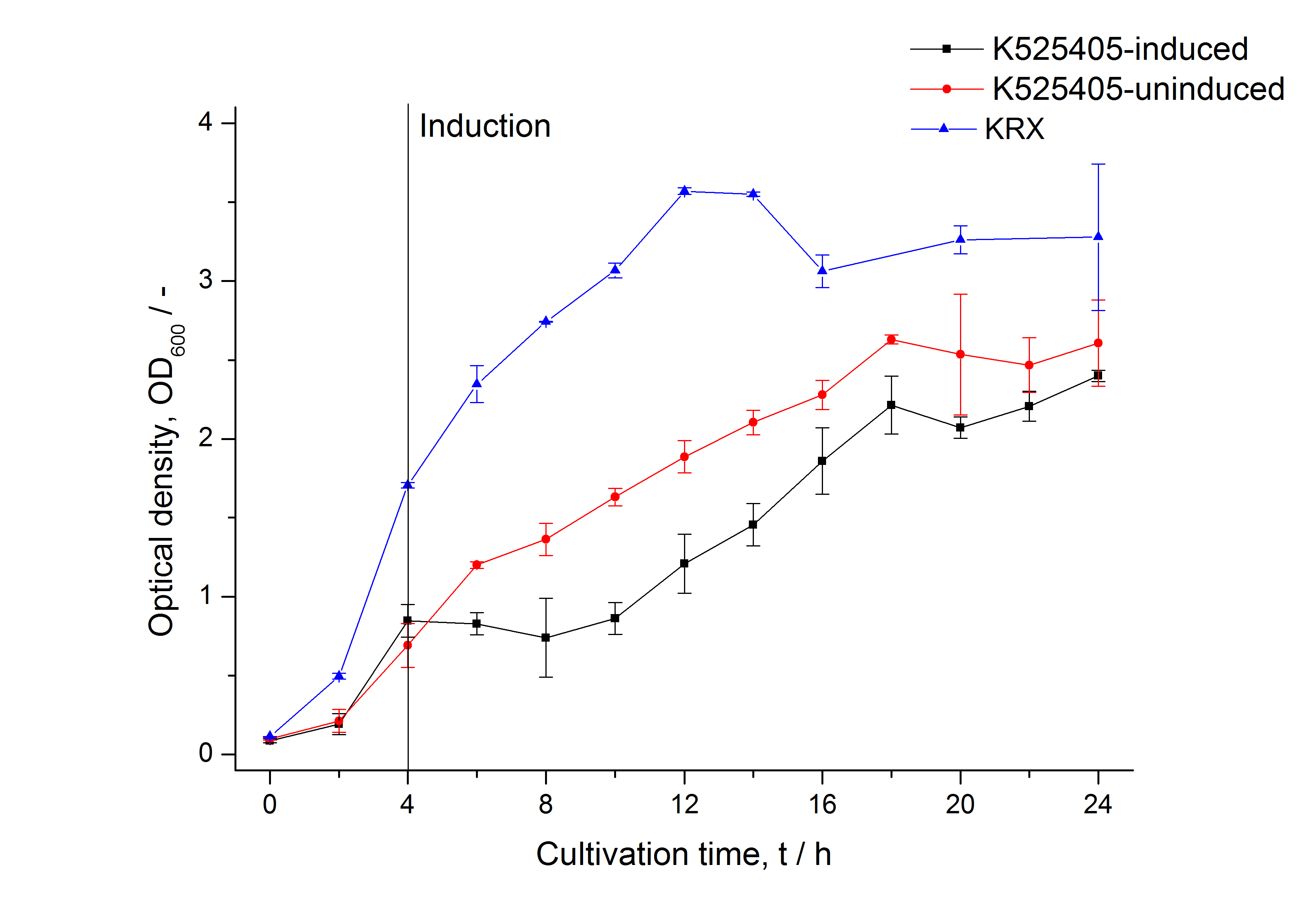
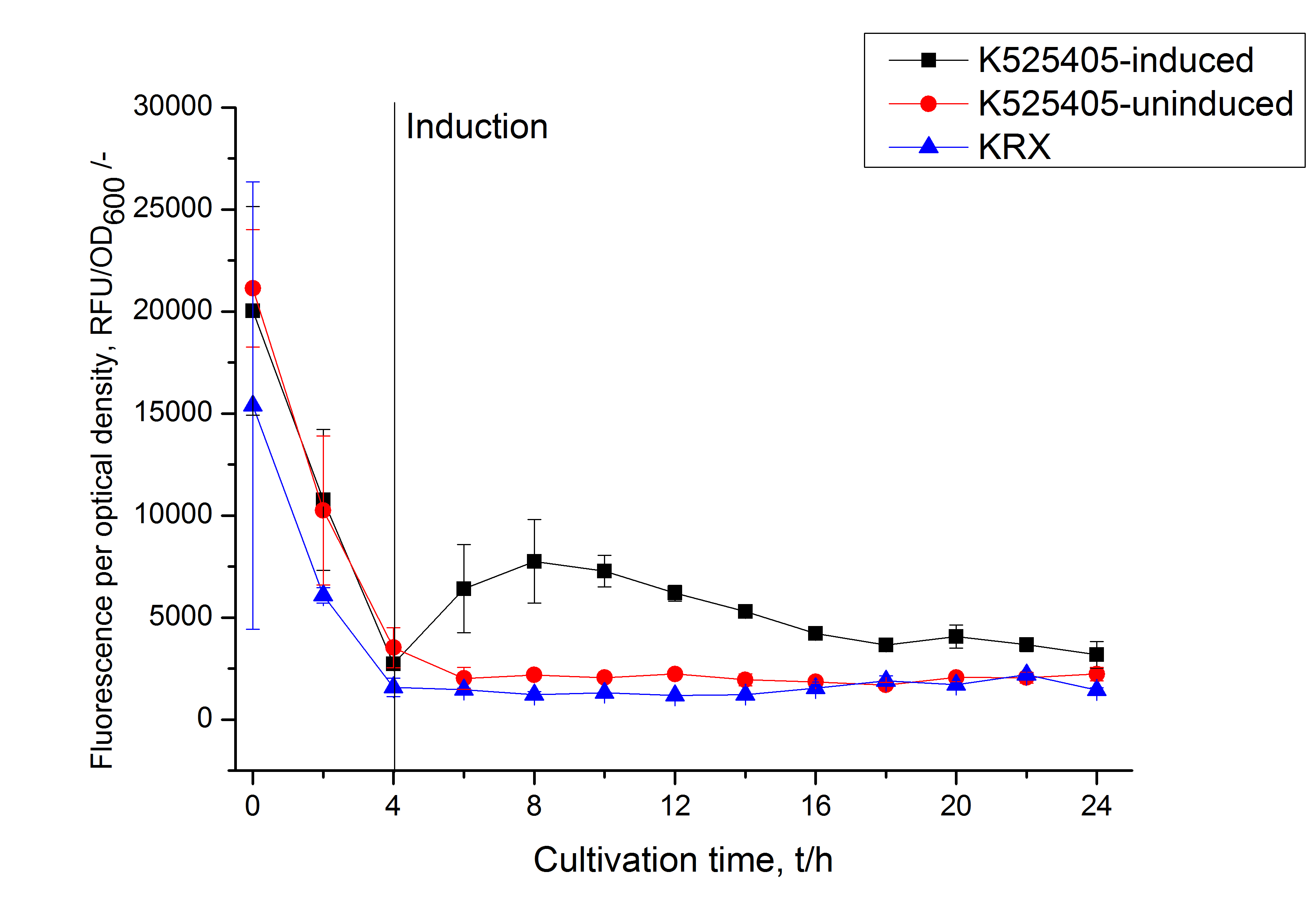
Purification of SbpA fusion protein
As seen in the analysis of the cultivations with expression of SbpA | mCitrine fusion proteins, these proteins form inclusion bodies in E. coli. Inclusion bodies have the advantage that they are relatively easy to clean-up and are resistant to proteases. So the first purification step is to solve and set-free the inclusion bodies. This step is followed by two filtrations (300 kDa UF and 100 kDa DF/UF) to further concentrate and purify the S-layer proteins. After the filtrations, the remaining protein solution is dialyzed against ddH2O for 18 h at 4 °C in the dark. The dialysis leads to a precipitation of the water-insoluble proteins. After centrifugation of the dialysate the water-soluble S-layer monomers remain in the supernatant and can be used for recrystallization experiments.
The fluorescence of some collected, important fractions of this purification strategy is shown in the following figure 3:
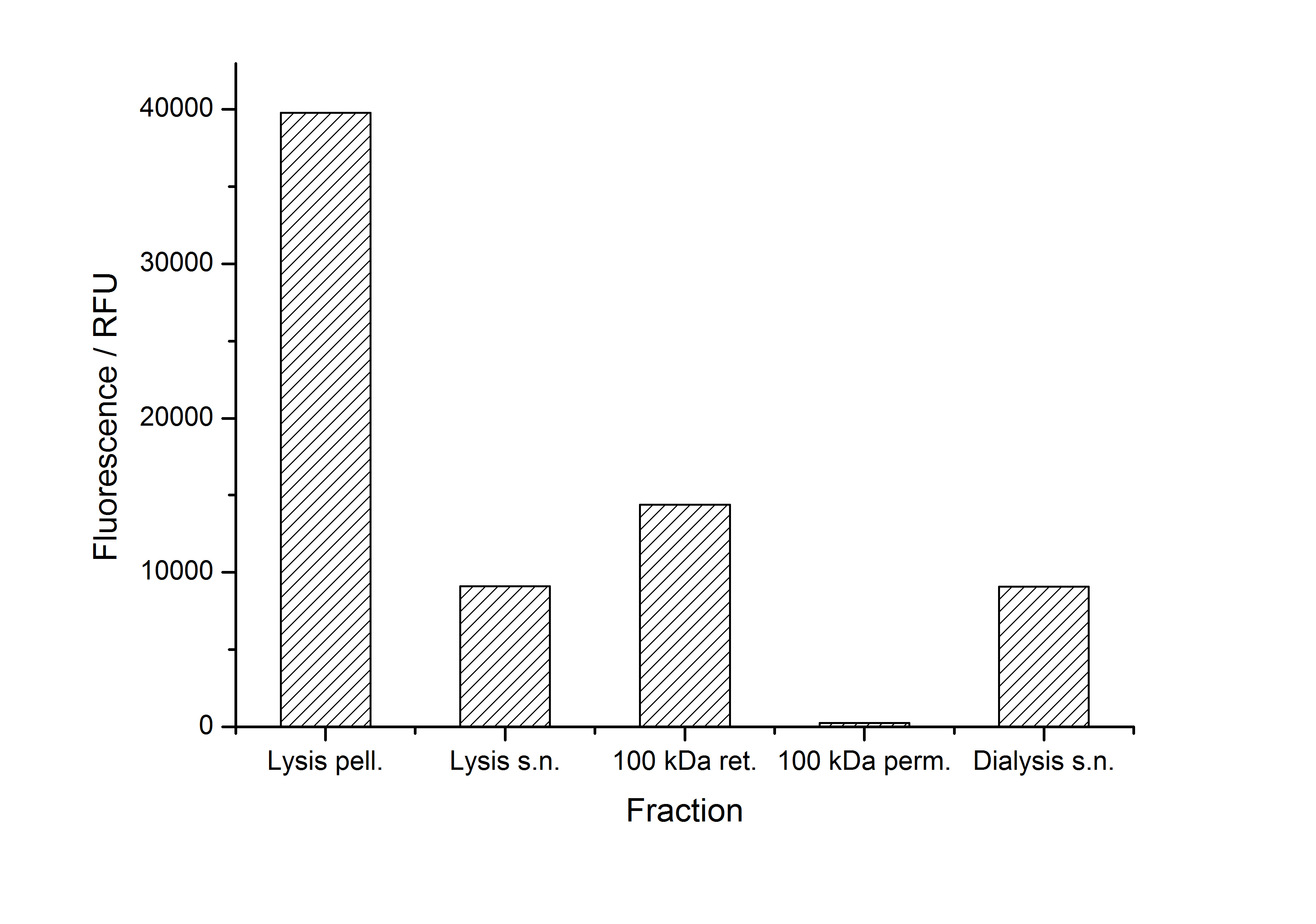
A lot of protein is lost during the purification especially after centrifugation steps (compared to filtrations). The fluorescence in the urea containing fractions is lowered due to denaturation of the fluorescent protein. This purification strategy is very simple and can be carried out by nearly everyone in any lab being one first step to enable real do it yourself nanobiotechnology.
Alternative purification strategy
Sometimes the inclusion body purification does not give very clean fractions. That's why we developed another purification strategy for BBa_K525405 monomers after inclusion body purification. This strategy consists of a capture with an IEX chromatography followed by a purification with HIC chromatography. Because of issues with the PES membranes we used to rebuffer our samples (see below - PES binds most of the S-layers) we lost a lot of our proteins in this step between the two chromatographies. So there was not enough protein left to stain it with Coomassie in a SDS-PAGE and time ran out to perform a silver staining. The fluorescence measurements of the collected fractions of the chromatographies are shown in the figures below.


Immobilization behaviour
After purification, solutions of monomeric SbpA S-layer proteins can be recrystallized and immobilized on silicon dioxide beads in recrystallization buffer (0.5 mM Tris-HCl, pH 9, 10 mM CaCl2). After the recrystallization procedure the beads are washed with and stored in ddH2O at 4 °C in the dark. The fluorescence of the collected fractions of recrystallization experiments with BBa_K525405 are shown in Fig. 6. 100 mg beads were coated with 100 µg of protein. The figure shows, that not all of the protein is immobilized on the beads (supernatant fraction) but the immobilization is pretty stable (very low fluorescence in the wash). After the immobilization, the beads show a high fluorescence indicating the binding of the SbpA | mCitrine fusion protein.
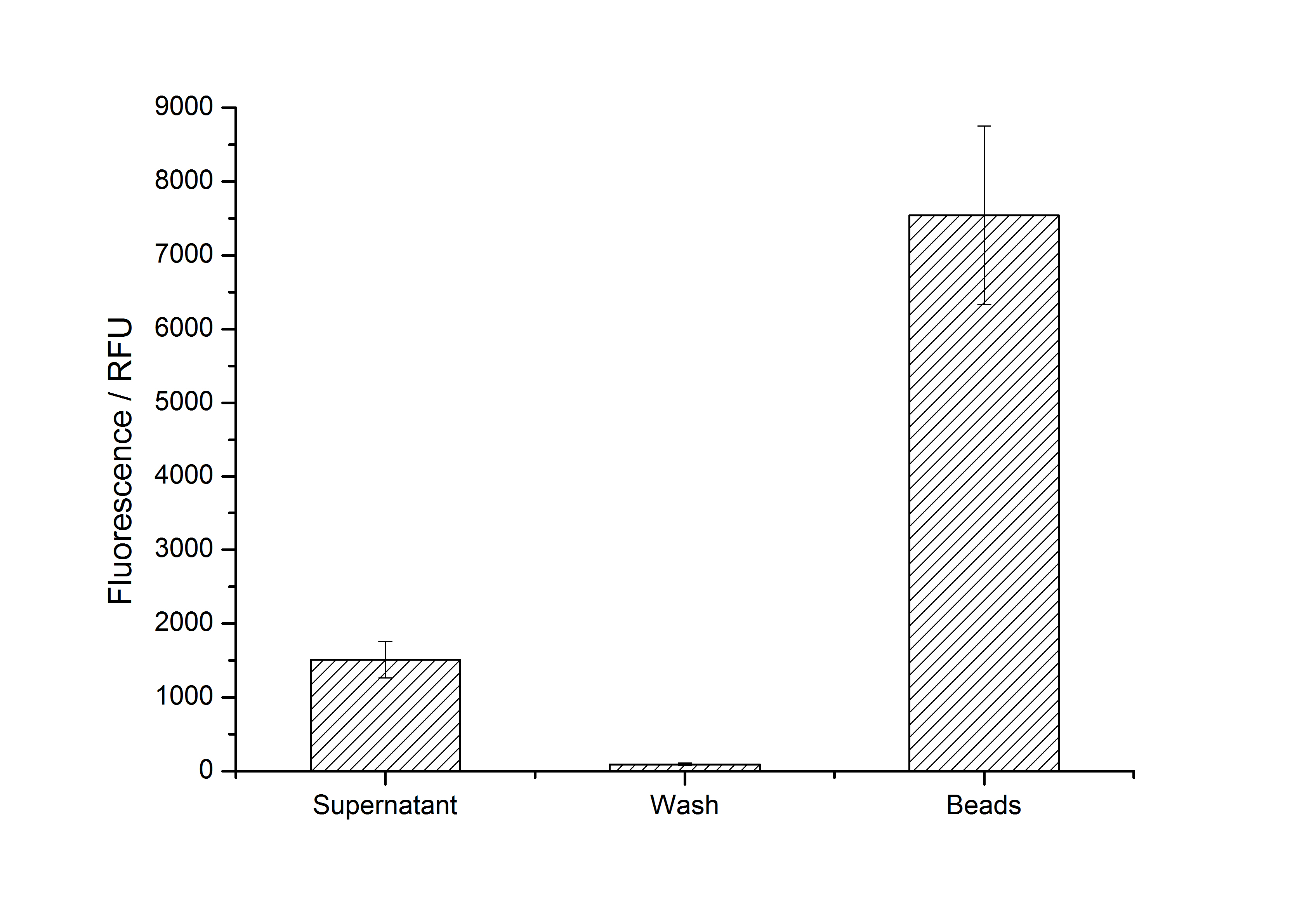
Optimal bead to protein ratio for immobilization
To determine the optimal ratio of silica beads to protein for immobilization, the degree of clearance ϕC in the supernatant is calculated and plotted against the concentration of silica beads used in the accordant immobilization experiment (compare Fig. 7):
The data was collected in three independent experiments. The fluorescence of the samples was measured in the supernatant of the immobilization experiment after centrifuging the silica beads. The fluorescence of the control was measured in a sample which was treated exactly like the others but no silica beads were added. 100 µg protein were used for one immobilization experiment. The data was fitted with a sigmoidal dose-response function of the form.
with the Hill coefficient p, the bottom asymptote A1, the top asymptote A2 and the switch point log(x0) (R² = 0.997).
The fit indicates that a good silica concentration for 100 µg of protein is 200 - 250 mg mL-1. This set-up leads to saturated beads with low waste of protein. So a good protein / bead ratio to work with is 7 - 9 * 10-4.
Methods
Expression of S-layer genes in E. coli in shaking flasks
- Chassis: Promega's [http://www.promega.com/products/cloning-and-dna-markers/cloning-tools-and-competent-cells/bacterial-strains-and-competent-cells/single-step-_krx_-competent-cells/ E. coli KRX]
- Medium: LB medium supplemented with 20 mg L-1 chloramphenicol
- For autoinduction: Cultivations in LB-medium were supplemented with 0.1 % L-rhamnose and 1 mM IPTG as inducer and 0.05 % glucose
- cultivation: 37 °C with 120 rpm in shaking flasks
Production of SgsE
- Cultivation
- Bioreactor: [http://www.bioengineering-inc.com/standard-reactors.php?id=2.1 Bioengineering NLF22 7 L] with Bioengineering DCU
- Medium: [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Materials#HSG_medium HSG medium] with 20 mg L-1 chloramphenicol
- Culture volume: 4 L
- Inoculation OD600: 0.2
- DO: 40 % airsaturation (controlled with stirrer cascade starting with 200 rpm)
- pH: 7.0 (controlled with 20 % phosphoric acid and 2 M NaOH)
- Induction after 4 h cultivation time with 2 % rhamnose and 0.1 mM IPTG (in culture medium)
- Harvest after 13 h
- Cell lysis
- Centrifuge down the cells (10000 g, 30 min, 4 °C)
- Resuspend pellet in enzyme buffer
- Cell lysis with high-pressure homogenizer (800 bar, 3 cycles at 4 °C)
- Centrifuge down the lysate (10000 g, 60 min, 4 °C)
- Inclusion body clean-up
- wash pellet from cell lysis with water twice
- after washing the pellet: incubate the pellet in [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Materials#Denaturation_buffer_for_inclusion_bodies denaturation buffer] for 60 min, 4 °C with vertical rotator
- final concentration in denaturation buffer: 0.5 mg wet biomass per mL
- centrifuge (60 min, >17000 g, 4 °C)
- in general with all centrifugations during this clean-up: the higher the speed, the better the result
- collect supernatant and incubate the pellet again in denaturation buffer (60 min, 4 °C, vertical rotator)
- centrifuge (60 min, >17000 g, 4 °C)
- collect supernatant and discard pellet

- Filtration
- Arrange the filtration module as shown on the right side.
- Collect permeate of cross flow filtration with 300 kDa membrane of sample before ultrafiltration
- This step is for removing cell debris
- Diafiltrate with 100 kDa membrane against [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Materials#Denaturation_buffer_for_inclusion_bodies denaturation buffer]
- constantly delute permeate with the buffer, keeping the permeate volume as low as possible
- Used membranes: [http://www.millipore.com/catalogue/module/C7493 Milipore Pellicon XL 50] or XL 100 membranes
- 50, 100 or 300 kDa cut-off
- 50 cm2 filtration area
- tangential flow filter
- Hydrophilic polyvinylidene fluoride membrane
- Used pump: SciLog TANDEM 1081 peristaltic pump
- flow rate during filtration: 40 mL min-1
- Dialysis
- Fill retentate from DF/UF in dialysis tube ([http://www.carl-roth.de/ Roth], cellulose, 10 kDa cut-off)
- Dialyse against ddH2O for 18 h at 4 °C in the dark
- After dialysis: centrifuge down the precipitation (45 min, 17000 g, 4 °C) and collect the supernatant
- Measure protein concentration in supernatant, dilute to 1 mg mL-1 with ddH2O and store at 4 °C in the dark
Measuring of mCitrine
- Take at least 500 µL sample for each measurement (200 µL is needed for one measurement) so you can perform a repeat determination
- Freeze biological samples at -80 °C for storage, keep cell-free at 4 °C in the dark
- To measure the samples thaw at room temperature and fill 200 µL of each sample in one well of a black, flat bottom 96 well microtiter plate (perform at least a repeat determination)
- Measure the fluorescence in a platereader (we used a [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader]) with following settings:
- 20 sec orbital shaking (1 mm amplitude with a frequency of 87.6 rpm)
- Measurement mode: Top
- Excitation: 488 nm
- Emission: 529 nm
- Number of reads: 25
- Manual gain: 100
- Integration time: 20 µs
References
Kainz B, Steiner K, Möller M, Pum D, Schäffer C, Sleytr UB, Toca-Herrera JL (2010) Absorption, Steady-State Fluorescence, Fluorescence Lifetime, and 2D Self-Assembly Properties of Engineered Fluorescent S-Layer Fusion Proteins of Geobacillus stearothermophilus NRS 2004/3a, [http://pubs.acs.org/doi/abs/10.1021/bm901071b Biomacromolecules 11(1):207-214].
Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM (2007) S-layers as a tool kit for nanobiotechnological applications, [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full FEMS Microbiol Lett 267(2):131-144].