Difference between revisions of "Help:Restriction enzymes"
| Line 1: | Line 1: | ||
[[Image:Enzymes.png|left]] | [[Image:Enzymes.png|left]] | ||
| + | Microbiologists and molecular biologists regularly use restriction enzymes to cut and splice DNA. | ||
| + | |||
| + | For example, if one wanted to move a piece of DNA from one plasmid to another, one could use restriction enzymes to cut it out of one plasmid, use restriction enzymes to create a gap in the destination plasmid, purify the desired segments, and mix the results. After ligation, if everything has worked properly, the DNA pieces will join together. | ||
| + | |||
| + | Restriction enzymes work by recognizing a particular sequence of bases on the DNA. The enzyme then cuts the DNA's backbones, allowing the DNA to separate into two pieces. | ||
| + | |||
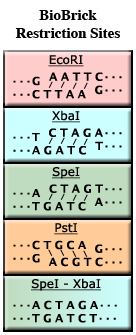
| + | For example, the enzyme EcoRI (see the figure, left) binds to the sequence GAATTC and cuts between the G and the A. It also cuts between the A and the G on the complementary strand. | ||
| + | |||
| + | '''Sticky Ends''' | ||
| + | |||
| + | Because the enzyme did not cut across both strands of DNA in the same place, four unmatched bases of DNA will ''stick out'' of each end (AATT in our example). These four bases of single-stranded DNA are said to be ''sticky'' in that they will stick to a matching piece of single stranded DNA. | ||
| + | |||
| + | If two pieces of DNA are cut by the same enzyme and then connected as described above, the result (after ligation) will be the original restriction site. If the same restriction enzyme is used again, the two pieces will be cut apart in the same way. However, since we want to be able to assemble two parts and then use the resulting assembly as a new part in the same process, we use two enzymes that have ''compatible'' sticky ends but ''incompatible'' recognition sequences. | ||
| + | |||
| + | '''SpeI - XbaI Mixed Sites''' | ||
| + | Note that both XbaI and SpeI have the same sticky ends, '''CTAG'''. As a result, DNA cut by one enzyme can stick to DNA cut by the other enzyme. However, after ligation, the resulting sequence does not match the recognition site of either XbaI or SpeI! This is called a '''mixed site''' and is designated with an "M". This is the basis for BioBrick assembly: by using pairs of resriction enzymes that produce mixed sites, like XbaI and SpeI, two BioBrick parts may be assembled; but once ligated, the result is a new part flanked by XbaI and SpeI, but with no internal XbaI or SpeI sites. | ||
| + | |||
| + | Because these four restriction enzymes are used to cut and manipulate BioBrick parts during the assembly process, BioBrick parts must not contain any of these four restriction sites. Registry tools check DNA sequences to enforce this requirement. | ||
| + | |||
| + | To learn more about restriction enzymes, see the New England Bioloabs website. | ||
Revision as of 17:22, 19 June 2006
Microbiologists and molecular biologists regularly use restriction enzymes to cut and splice DNA.
For example, if one wanted to move a piece of DNA from one plasmid to another, one could use restriction enzymes to cut it out of one plasmid, use restriction enzymes to create a gap in the destination plasmid, purify the desired segments, and mix the results. After ligation, if everything has worked properly, the DNA pieces will join together.
Restriction enzymes work by recognizing a particular sequence of bases on the DNA. The enzyme then cuts the DNA's backbones, allowing the DNA to separate into two pieces.
For example, the enzyme EcoRI (see the figure, left) binds to the sequence GAATTC and cuts between the G and the A. It also cuts between the A and the G on the complementary strand.
Sticky Ends
Because the enzyme did not cut across both strands of DNA in the same place, four unmatched bases of DNA will stick out of each end (AATT in our example). These four bases of single-stranded DNA are said to be sticky in that they will stick to a matching piece of single stranded DNA.
If two pieces of DNA are cut by the same enzyme and then connected as described above, the result (after ligation) will be the original restriction site. If the same restriction enzyme is used again, the two pieces will be cut apart in the same way. However, since we want to be able to assemble two parts and then use the resulting assembly as a new part in the same process, we use two enzymes that have compatible sticky ends but incompatible recognition sequences.
SpeI - XbaI Mixed Sites Note that both XbaI and SpeI have the same sticky ends, CTAG. As a result, DNA cut by one enzyme can stick to DNA cut by the other enzyme. However, after ligation, the resulting sequence does not match the recognition site of either XbaI or SpeI! This is called a mixed site and is designated with an "M". This is the basis for BioBrick assembly: by using pairs of resriction enzymes that produce mixed sites, like XbaI and SpeI, two BioBrick parts may be assembled; but once ligated, the result is a new part flanked by XbaI and SpeI, but with no internal XbaI or SpeI sites.
Because these four restriction enzymes are used to cut and manipulate BioBrick parts during the assembly process, BioBrick parts must not contain any of these four restriction sites. Registry tools check DNA sequences to enforce this requirement.
To learn more about restriction enzymes, see the New England Bioloabs website.