Difference between revisions of "Part:BBa K574009:Experience"
CubicStone (Talk | contribs) (→Applications of BBa_K574009) |
CubicStone (Talk | contribs) (→Applications of BBa_K574009) |
||
| Line 9: | Line 9: | ||
1. Ligate E0840 (''<partinfo>E0840</partinfo>'') downstream of K574009, which acts as a reporter. <br> | 1. Ligate E0840 (''<partinfo>E0840</partinfo>'') downstream of K574009, which acts as a reporter. <br> | ||
2. Culture the positive colony at 37°C, 220 rpm for 12 hours, as well as the DH5alpha. <br> | 2. Culture the positive colony at 37°C, 220 rpm for 12 hours, as well as the DH5alpha. <br> | ||
| − | 3. Dilute 1:25 the former in | + | 3. Dilute 1:25 the former in 15 falcon tubes (4 ml cultures), each 3 tubes as a group. The later is also diluted in 3 tubes as the negative control. <br> |
| − | 4. After culturing for another 2 hours, as their OD600 reach 0.2, induce with 3OC12HSL 0, 1e-6 M, 1e-5 M and 1e-4 M respectively. <br> | + | 4. After culturing for another 2 hours, as their OD600 reach 0.2, induce with 3OC12HSL 0, 1e-7 M, 1e-6 M, 1e-5 M and 1e-4 M respectively. <br> |
5. Take samples after 1 hour, 4 hours, 8 hours and 24 hours, that is, centrifugalizate 0.5 mL of each culture and suspend them by 1 mL of PBS (phosphate-buffered saline). <br> | 5. Take samples after 1 hour, 4 hours, 8 hours and 24 hours, that is, centrifugalizate 0.5 mL of each culture and suspend them by 1 mL of PBS (phosphate-buffered saline). <br> | ||
6. Measure the fluorescence of the samples with the flow cytometer. | 6. Measure the fluorescence of the samples with the flow cytometer. | ||
Latest revision as of 01:44, 6 October 2011
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K574009
Description: we assembled E0840 (BBa_E0840) under the pLas promoter (BBa_R0079) that was contained into K574009. We kept pSB1A2 as the scaffold vector.
Methods:
1. Ligate E0840 (BBa_E0840) downstream of K574009, which acts as a reporter.
2. Culture the positive colony at 37°C, 220 rpm for 12 hours, as well as the DH5alpha.
3. Dilute 1:25 the former in 15 falcon tubes (4 ml cultures), each 3 tubes as a group. The later is also diluted in 3 tubes as the negative control.
4. After culturing for another 2 hours, as their OD600 reach 0.2, induce with 3OC12HSL 0, 1e-7 M, 1e-6 M, 1e-5 M and 1e-4 M respectively.
5. Take samples after 1 hour, 4 hours, 8 hours and 24 hours, that is, centrifugalizate 0.5 mL of each culture and suspend them by 1 mL of PBS (phosphate-buffered saline).
6. Measure the fluorescence of the samples with the flow cytometer.
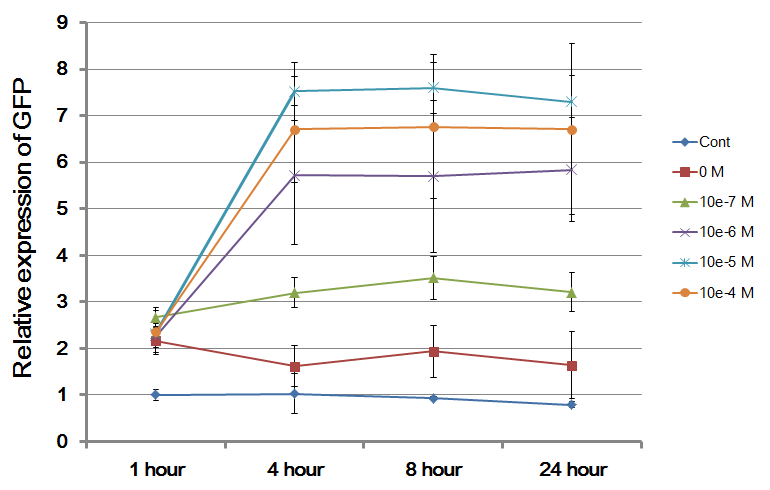
Results: From the chart, we can see that cells were hardly induced in the control group, and with the concentration of inducer growing, the intensity of GFP increased by groups. The most efficient concentration of inducer was around 10^-5M, and higher concentration may lead to the expression of GFP decreasing. Additionally, to most groups, the intensity of GFP reached its maxium after 4 hours.
User Reviews
UNIQdd403c3ce0c48b84-partinfo-00000003-QINU UNIQdd403c3ce0c48b84-partinfo-00000004-QINU
