Difference between revisions of "Part:BBa K554005"
Neshich.iap (Talk | contribs) (→Usage and Biology) |
Neshich.iap (Talk | contribs) (→Usage and Biology) |
||
| Line 17: | Line 17: | ||
<!-- --> | <!-- --> | ||
| − | ===<font size+5>Usage and Biology</font>=== | + | ===<font size +5>Usage and Biology</font>=== |
==A fusion IL-12 protein== | ==A fusion IL-12 protein== | ||
Revision as of 05:38, 22 September 2011
IL12
IL12 is an heterodimeric cytokine, composed of a disulfide-bound 35 kDa subunit (p35) and a 40 kDa subunit (p40) which enhances both innate and adaptative immune system. It is primarily produced by antigen-presenting cells such as dentritic cells.MORALES et al. (2004) established that sustained IL-12 signaling is required for Th1 development: It drives naïve Th0 differentiation towards the Th1 phenotype, which makes it an appropriate answer to a Th2 imbalance. We thus decided to create a completely new biobrick, responsible for Il-12 synthesis.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1409
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
A fusion IL-12 protein
- As MORRIS et al. (2008) [4] recollect, “Early approaches in generating recombinant IL-12 involved co-transfection of cells with two different vectors, each expressing one of the two genes (p40 or p35), or expression of the individual genes under the control of separate promoters in a single plasmid or viral vector. Both approaches were disadvantaged due to inefficient expression of the functional heterodimer with the likelihood of p40 homodimer production” (And thus competitive IL-12 antagonist action.)
- In order to counter those difficulties, several research teams reported the expression of a bioactive single chain porcine IL-12 fusion protein, such as LIESCHKE et al. (1997), whose protein comprised the p40 subunit linked by a (Gly, Ser) linker to the p35 subunit from which the first 22 amino acids were deleted. It proved bioactive with an apparent specific activity comparable to recombinant human IL-12.
Our IL-12 sequence
- As successful work (correct synthesis and protein proved bioactive) has already been done on it , we chose to use a fusion construct of human IL-12, similarly as the one used by LIESCHKE et al. (1997) porcine fusion IL-12 protein for our project.
A synthesis trigger: promoter flhDC
- We need a device that only works when needed: recombinant IL-12 expression must not be continuous, it has to be a reaction to a Th2 imbalance and stop when the balance is reestablished. Therefore, we need a promoter that would be a sensible “trigger”.
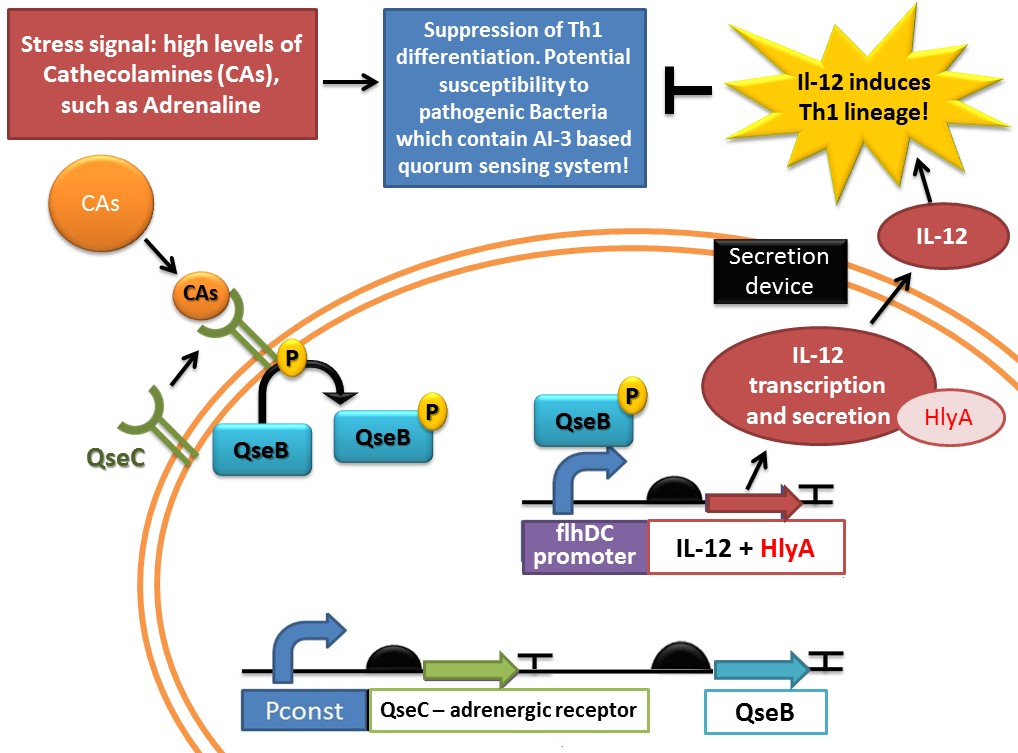
- As we explained in the sensor part (the bacterial adrenergic sensor), our device will detect a Th2 imbalance via QseB/QseC sensor, that will be activated in response to adrenaline detection. It will then be able to activate the flhDC promoter (SPERANDIO et al. , 2002) that we will be used to control the IL-12 gene expression.
IL-12 secretion
- With this right promoter, a ribosome binding site (RBS), the IL-12 gene, and a terminator, E.Coli is now able to synthesize IL-12 in response to a Th2 imbalance. But, being a bacteria, it doesn’t have the required material to secrete it in the body. Hence we also need to construct a secretion system, that will be described in the dedicated part of this wiki.
- In our “IL-12 synthesis part”, we will just add a secretion signal sequence (HlyA) for IL-12 to be correctly dealt with by the secretion system.
This sequence is used by [http://2011.igem.org/Team:UNICAMP-EMSE_Brazil UNICAMP-EMSE Brazil team] in the [http://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project/Device1 Adrenaline sensor device] ("Device 1", which senses Cathecolamine and AI-3 levels and responds by producing and secreting IL-11. This part is shown in red in the following schema:
References
- Hidalgo E, Leautaud V, Demple B (1998) The redox-regulated SoxR protein acts from a single DNA site as a repressor and an allosteric activator. The EMBO Journal 17(9)2629–2636 [http://www.ncbi.nlm.nih.gov/pubmed/?term=9564045%20 Link to Pubmed]